 |
 |
 |
| |
Results of Patient-Reported Outcome Data from the Phase III BRIGHTE Study of Fostemsavir
|
| |
| |
Reported by Jules Levin
IDWeek 2018, October 3-7, 2018, San Francisco
from Jules: from several sources overall viral suppression among HIV+ in the USA is 55% and adherence is sub par too. These individuals as they age are subject to bad outcomes in quality of life as well as aging related syndrome underpinning & reinforcing the affects of aging & HIV, these individuals are likely to have worse mental & physical function, more comorbidities & be disabled. Its a false notion promoted that today is different than for older HIV+ who started ART with low nadir CD4s so today with recommended ART at 500 or greater CD4 this is not likely to change the overall affects on aging & HIV very much. Even those who start with CD4 over 500 its the HIV that causes aging, immuno-senescence & inflammation & immune activation which leads to the aging syndrome & multiple comorbidities including frailty etc. not just the low nadir CD4.
Clare Proudfoot, PhD1, Peter Ackerman, MD2, Cyril Llamoso, MD2, David Cella, PhD3, Andrew Clark, MD1 and Miranda Murray, PhD1, (1)ViiV Healthcare UK Ltd, Brentford, United Kingdom, (2)ViiV Healthcare, Branford, CT, (3)Medical Social Sciences, Northwestern, Chicago, IL
EACS-2017: Phase 3 Study of Fostemsavir in Heavily Treatment-Experienced HIV-1-Infected Participants: Day 8 and Week 24 Primary Efficacy and Safety Results (BRIGHTE Study, Formerly 205888/AI438-047) - (10/30/17)
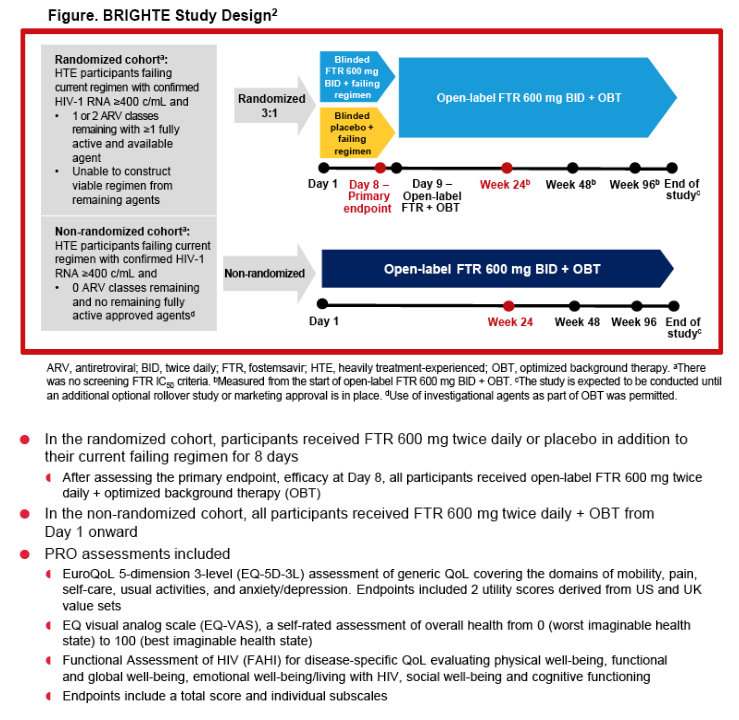
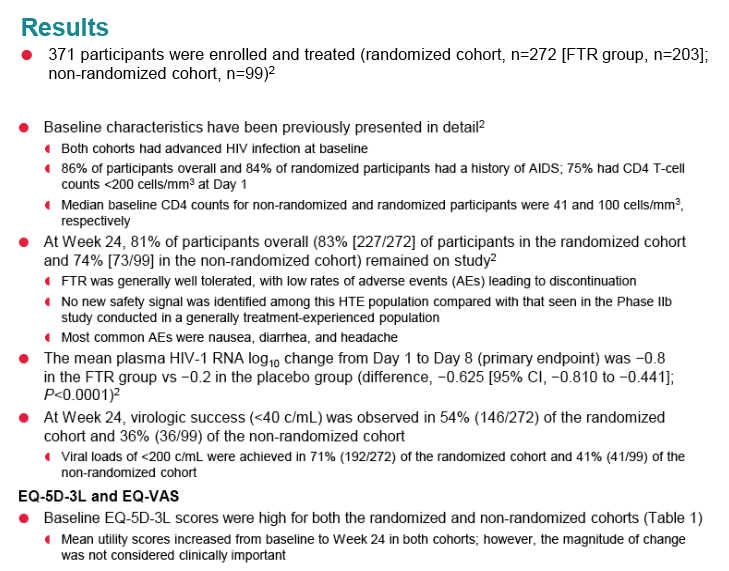
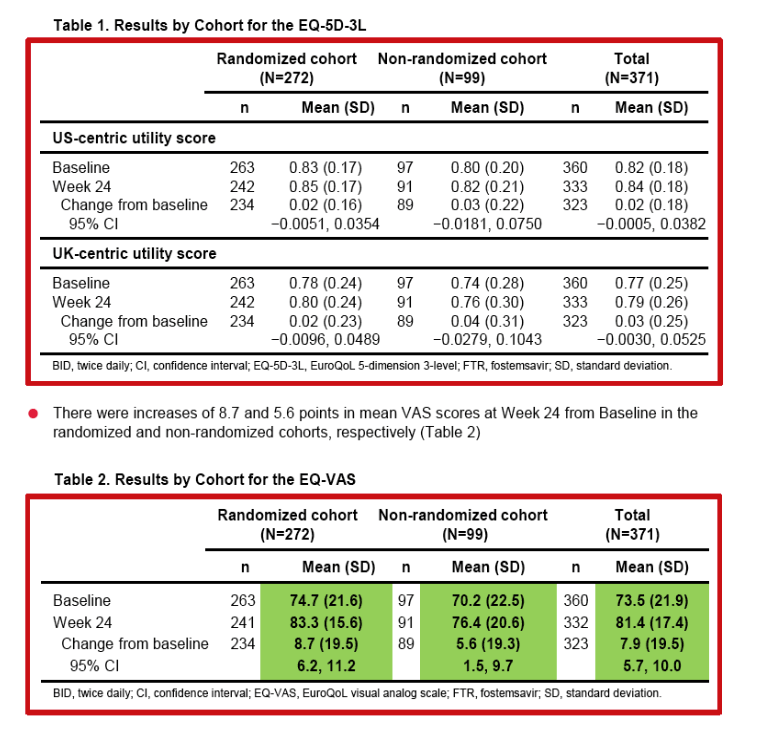

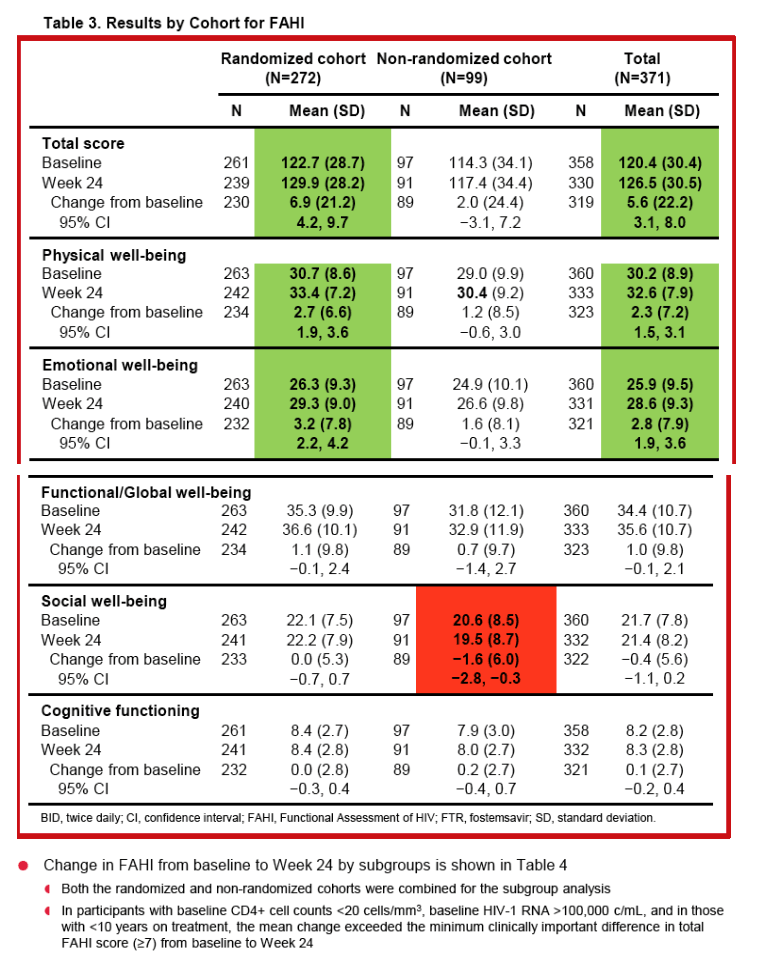
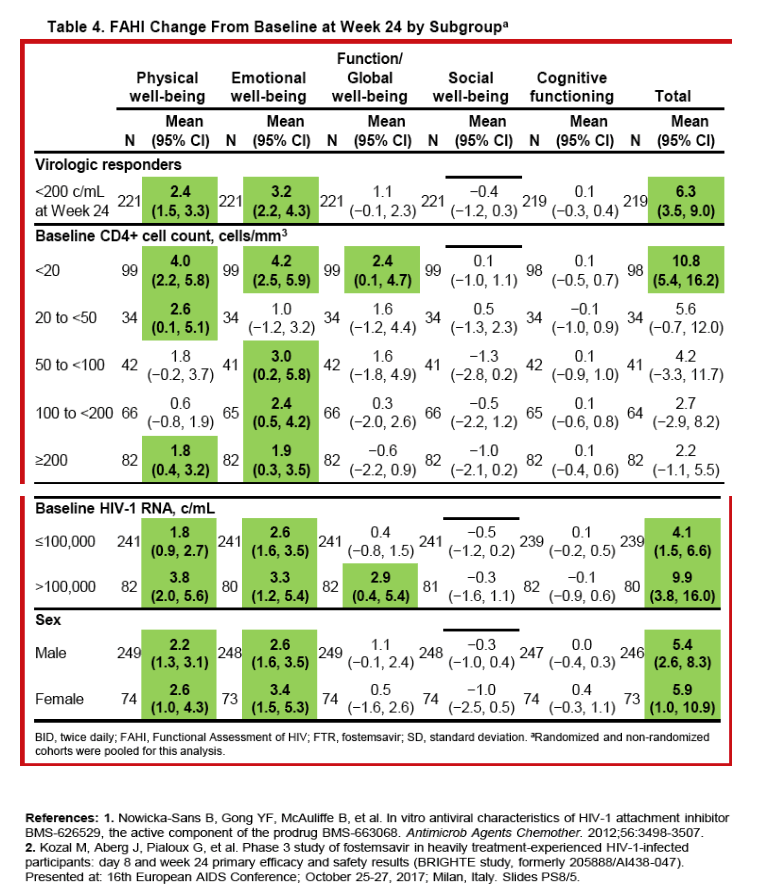
|
| |
|
 |
 |
|
|