 |
 |
 |
| |
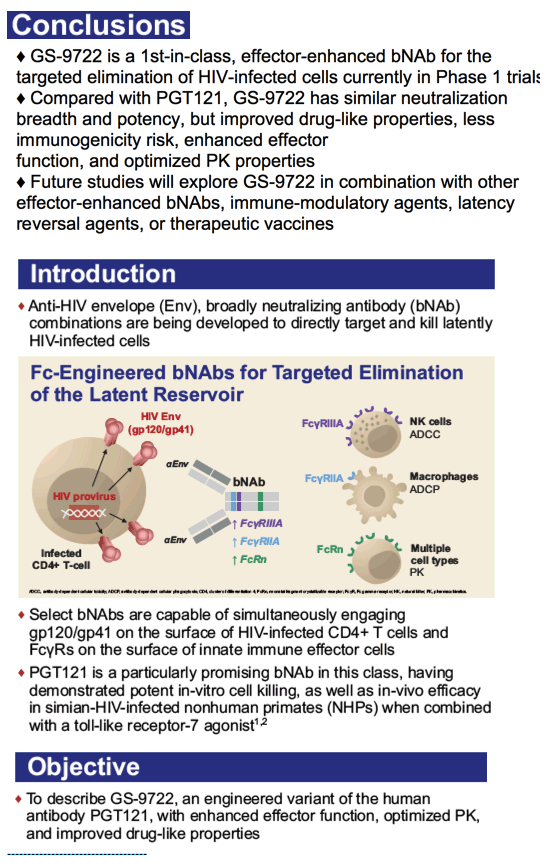
GS-9722: First-in-Class, Effector-Enhanced, Broadly Neutralizing
Antibody for HIV Cure - and PGT121 in HIV+ / PGT121 bNAb in HIV+
|
| |
| |
THERAPEUTIC ACTIVITY OF PGT121 MONOCLONAL ANTIBODY IN HIV-INFECTED ADULTS
see below oral at CROI in HIV+ as treatment.
Long-acting treatment with a broadly neutralizing antibody plus long-acting ART is a concept to consider. Jules
Reported by Jules Levin
CROI 2019 March 4-7 Seattle

-----------------------------------
THERAPEUTIC ACTIVITY OF PGT121 MONOCLONAL ANTIBODY IN HIV-INFECTED ADULTS (ABSTRACT 145)
Kathryn E. Stephenson
Beth Israel Deaconess Medical Center, Boston, MA, USA
In this Phase 1 study, PGT121 was safe and well-tolerated with a favorable PK profile. PGT121 led to a median 1.7 log drop in VL in viremic HIV+ participants with PGT121-sensitive virus and high VL at baseline. Surprisingly, a single infusion of PGT121 led to >5 months of viral suppression to < lower limit of quantification in 2 participants with the lowest VL at baseline. Follow up is ongoing to determine PGT121-induced changes to host immunity, as well as the ultimate duration of ART-free virologic remission in suppressed participants.
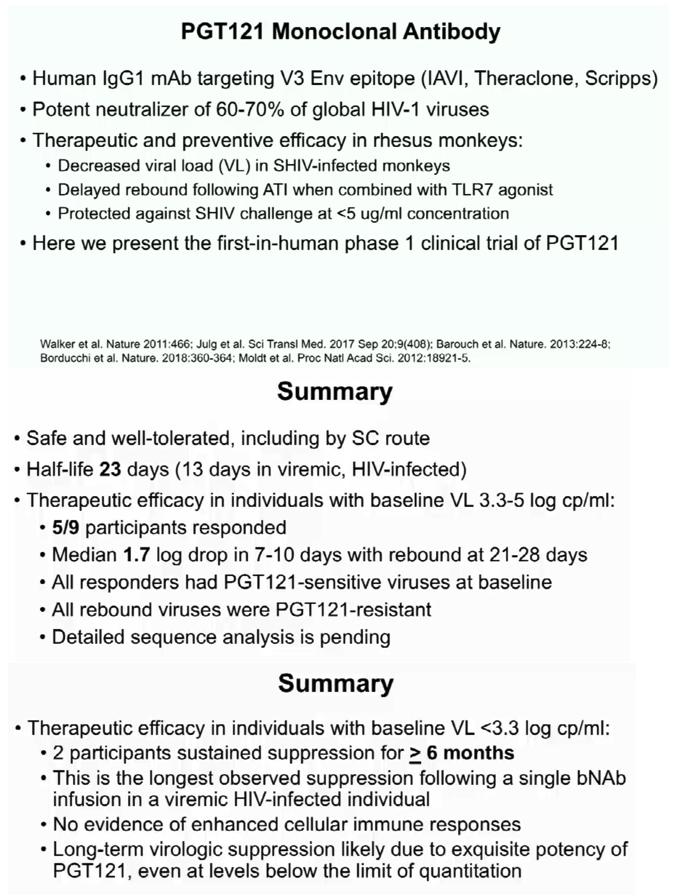
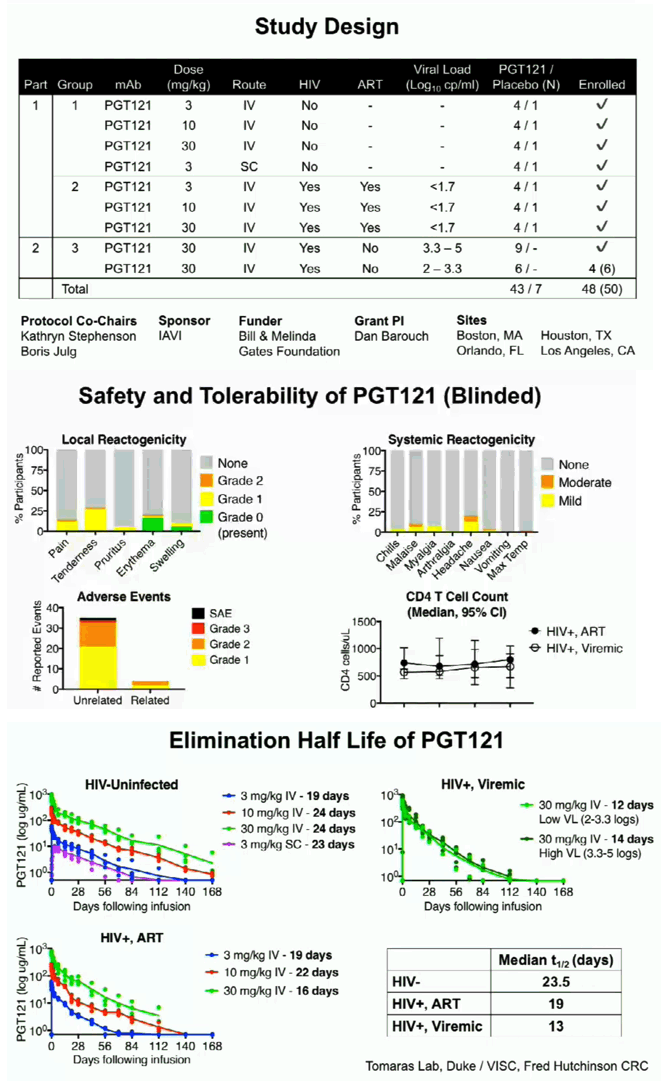
The first part of the study was a randomized, double blinded, dose escalation, placebo-controlled trial of PGT121 in adults who were HIV-uninfected (HIV-, N=20) and HIV-infected on ART (HIV+/ART+, N=15). PGT121 was given once at 3, 10, and 30 mg/kg IV and 3 mg/kg SC (N=5/group, 4:1 Ab/placebo). The second part of the study was an open label trial of PGT121 given once at 30 mg/kg IV in HIV-infected adults not on ART with high VL (3.3-4.8 log cp/ml, N=9) and low VL (2-2.6 log cp/ml, N=3). All participants were monitored for reactogenicity for 3 days and adverse events (AEs) for 56 days. PK and virologic assessments were performed through 6 months. The lower limit of quantification (LLOQ) of VL was 1.6 log cp/ml.
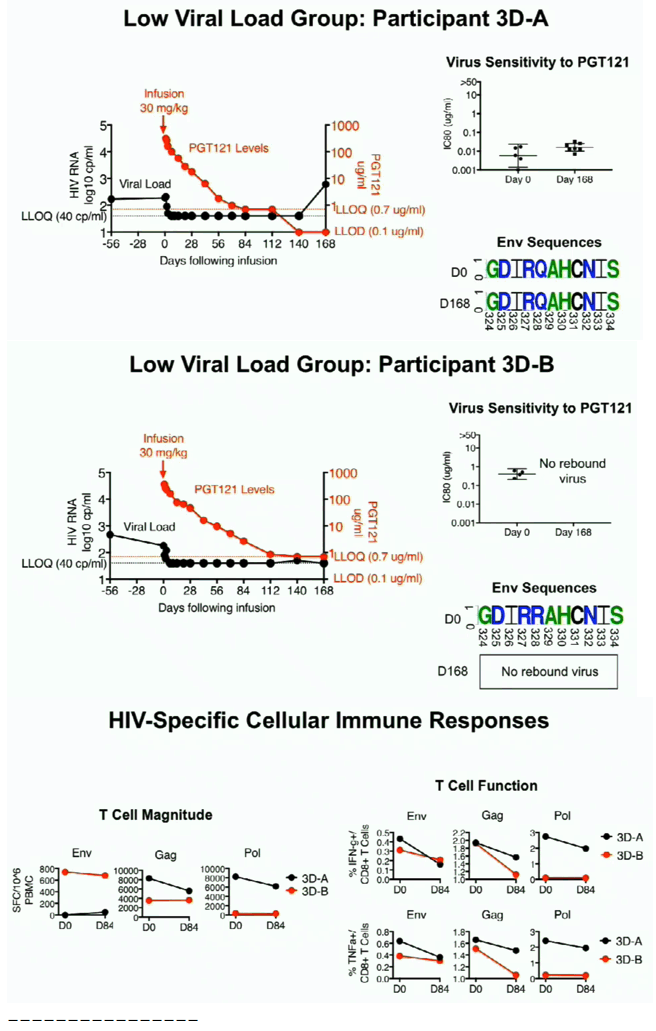
PGT121 was safe and well-tolerated with no related mod/severe AEs. The elimination half-life of PGT121 was ∼22 days in HIV- and HIV+/ART+ groups, with variation by dose and route. In viremic HIV+ individuals not on ART, PGT121 showed antiviral efficacy in 5/9 participants in the high VL group with a median drop in VL of 1.7 log cp/ml (1.3-2.1) by d10. These individuals showed PGT121 sensitive virus at baseline but developed rebound by d28 with emergence of resistance. In the low VL group, PGT121 decreased VL to < LLOQ by d7 in 3/3 participants. Two of these individuals showed prolonged ART-free virologic suppression following 1 infusion with PGT121, with 1 individual experiencing rebound only at 6 months. The second individual's VL is still < LLOQ at 6 months, despite PGT121 levels that have declined to <0.86 ug/ml.
http://www.croiwebcasts.org/console/player/41314?mediaType=slideVideo&&crd_fl=0&ssmsrq=1552667780672&ctms=5000&csmsrq=5049


|
| |
|
 |
 |
|
|