 |
 |
 |
| |
MK-8591 Potency and PK Provide High
Inhibitory Quotients at Low Doses QD and QW
|
| |
| |
Reported by Jules Levin
CROI 2019 March 4-7 Seattle
Jay A. Grobler1; Kerry L. Fillgrove2; Daria J. Hazuda1; Qian Huang1; Ming-Tain Lai1; Randolph P. Matthews3; Deanne J. Rudd2; Ryan C. Vargo2
Departments of 1Infectious Disease and Vaccines; 2PPDM; 3Translational Pharmacology;Merck & Co., Inc., Kenilworth, NJ, USA
Abstract
Background: MK-8591, a nucleoside reverse transcriptase translocation inhibitor (NRTTI), has demonstrated HIV-1 suppression for ≥7 days with single doses as low as 0.5 mg. It is currently in a Phase 2 clinical trial (NCT03272347) for the treatment of HIV-1 infection with once-daily (QD) administration of 0.25 mg, 0.75 mg, or 2.25 mg in combination with doravirine. Inhibitory quotients (IQ) for nucleoside inhibitors, based on the ratio of intracellular phosphorylated drug concentrations at trough (Ctrough,IC) and the intracellular concentrations required for efficacy (IC50,IC), predict virologic response. We evaluated the IQ of MK-8591-triphosphate (MK-8591-TP) in relation to other NRTIs for WT and NRTI-resistant HIV-1 to assess the likelihood of virologic response and barrier to resistance at clinically relevant doses.
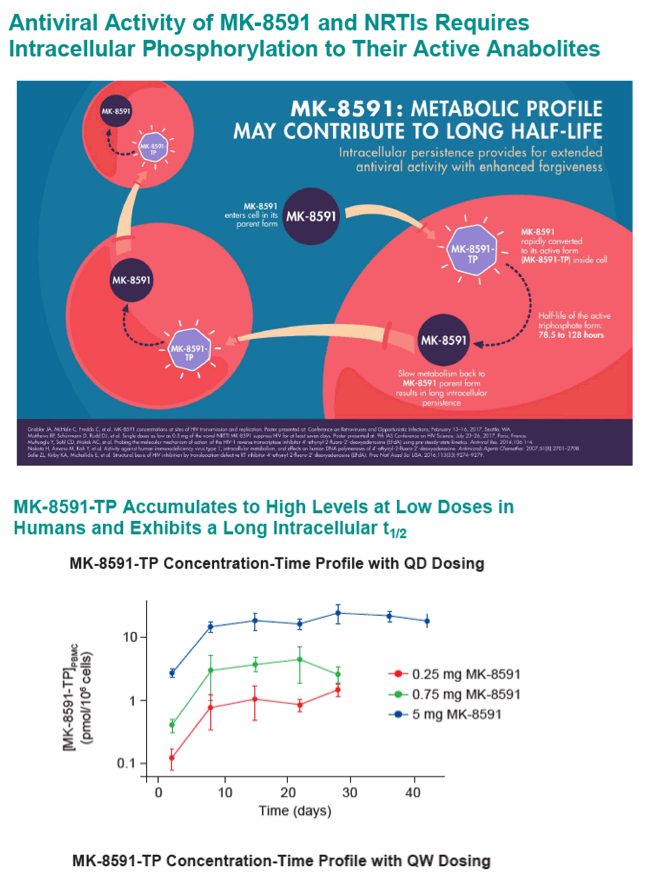
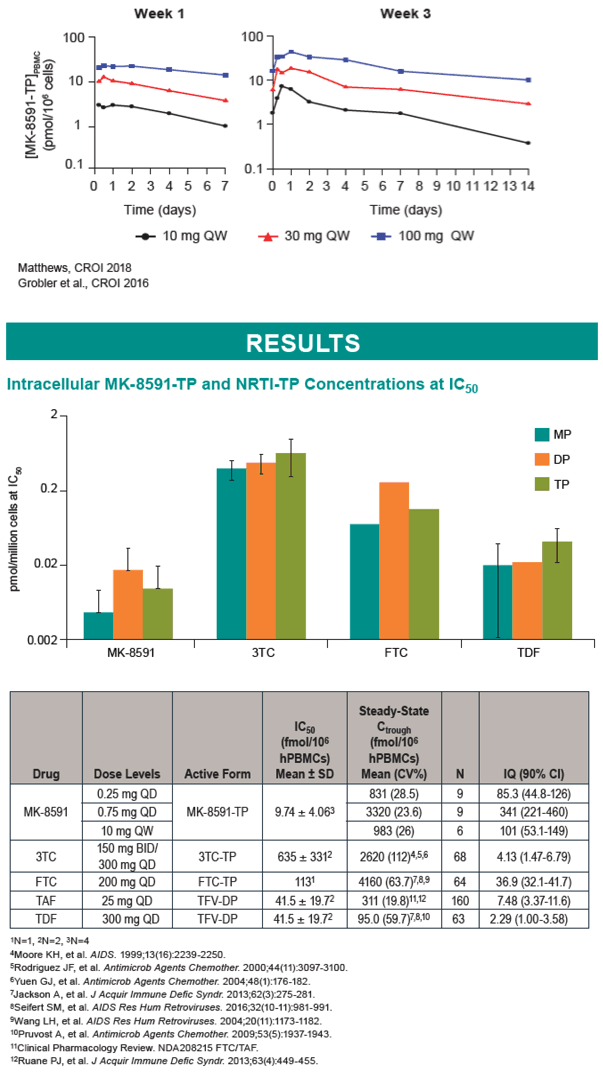
Methods: MK-8591-TP, TFV-DP, 3TC-TP, and FTC-TP IC50,IC levels were determined in activated, uninfected human peripheral blood mononuclear cells (hPBMC) after 24 hr incubation with varying concentrations of MK-8591, TDF, 3TC, or FTC, followed by lysis and analysis by LC-MS/MS. MK-8591 IQs for wild-type (WT) HIV-1 were calculated as the ratio of steady-state Ctrough,IC, as observed with QD or weekly (QW) dosing in Phase 1 clinical studies, to the IC50,IC in hPBMCs. TDF, TAF, 3TC, and FTC IQs were calculated using their corresponding Ctrough,ICs, as determined after dosing in humans at clinical dose levels, and hPBMC IC50,ICs. IQs for NRTI-resistant HIV-1 were calculated using fold-shifts for NRTI-resistant clinical isolates.
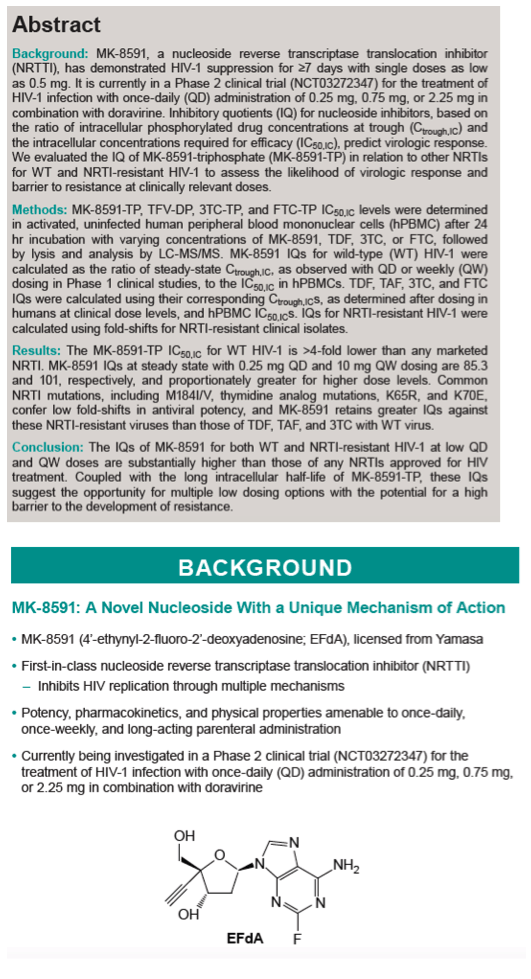
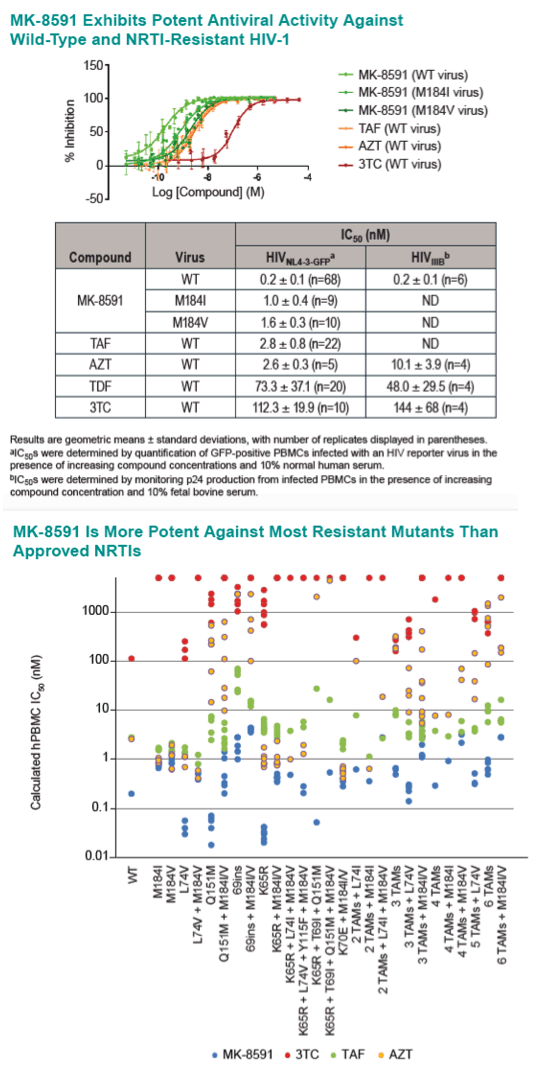
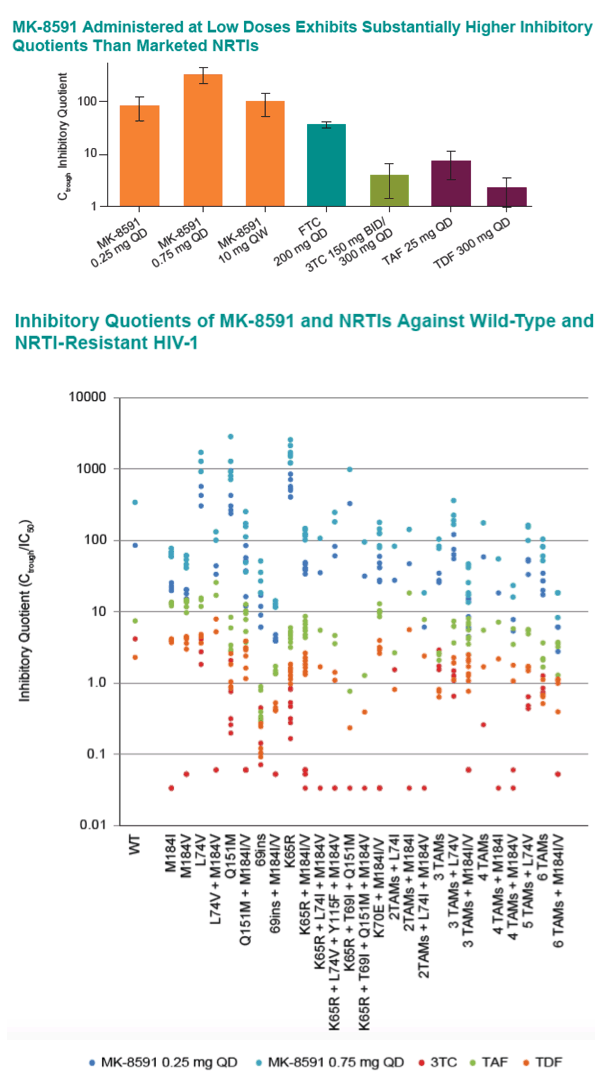
Results: The MK-8591-TP IC50,IC for WT HIV-1 is >4-fold lower than any marketed NRTI. MK-8591 IQs at steady state with 0.25 mg QD and 10 mg QW dosing are 85.3 and 101, respectively, and proportionately greater for higher dose levels. Common NRTI mutations, including M184I/V, thymidine analog mutations, K65R, and K70E, confer low fold-shifts in antiviral potency, and MK-8591 retains greater IQs against these NRTI-resistant viruses than those of TDF, TAF, and 3TC with WT virus.
Conclusion: The IQs of MK-8591 for both WT and NRTI-resistant HIV-1 at low QD and QW doses are substantially higher than those of any NRTIs approved for HIV treatment. Coupled with the long intracellular half-life of MK-8591-TP, these IQs suggest the opportunity for multiple low dosing options with the potential for a high barrier to the development of resistance.
|
| |
|
 |
 |
|
|