 |
 |
 |
| |
Modest 96-Week Weight Gain in Trials of Doravirine, Darunavir, Efavirenz
"EFFECT OF DORAVIRINE ON BODY WEIGHT AND BODY MASS INDEX IN TREATMENT NAÏVE ADULTS WITH HIV-1"
|
| |
| |
17th European AIDS Conference, November 6-9, 2019, Basel
Mark Mascolini
Previously untreated adults starting the nonnucleoside doravirine in 3 randomized comparisons with darunavir/ritonavir or efavirenz gained an average 2.4 kg through 96 weeks, not much more than the 1.8-kg uptick seen with darunavir or the 1.6-kg bump with efavirenz [1]. With all three regimens, 13% to 17% of participants had a 10% or greater weight gain through 96 weeks.
Recent trials of HIV integrase inhibitors raised concern of excessive weight gain with these agents, particularly when given with tenofovir alafenamide (TAF). After 96 weeks in the South African ADVANCE trial, also presented at the 17th European AIDS Conference [2] and summarized separately by NATAP, people randomized to the integrase inhibitor dolutegravir gained more weight than those randomized to efavirenz, and people assigned to TAF added more pounds than those assigned to tenofovir disoproxil fumarate (TDF) [2]. In several studies, women and blacks gained more weight than men and nonblacks.
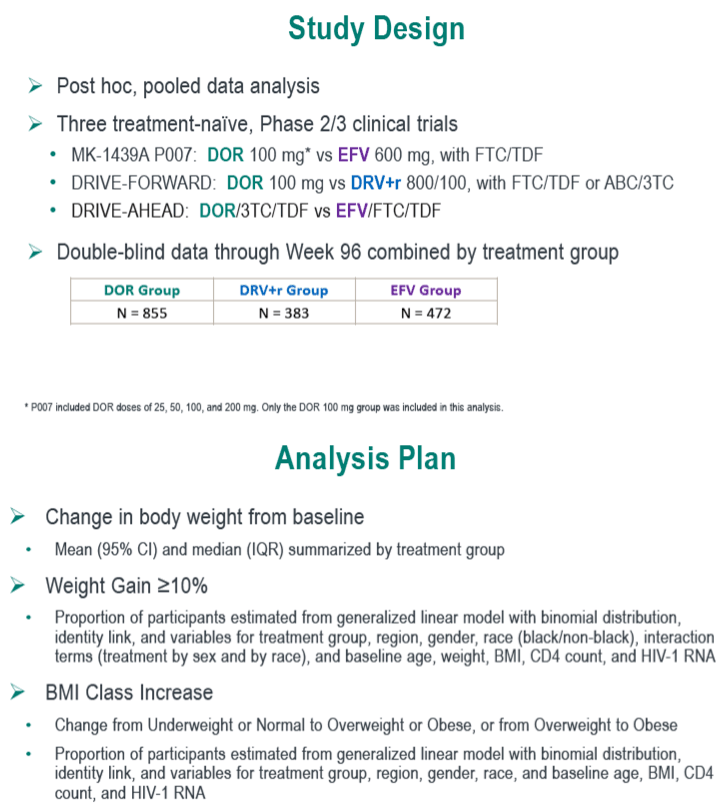
Doravirine is a nonnucleoside licensed as a stand-alone antiretroviral for combination therapy and as a 3-in-1 pill with TDF and lamivudine (3TC). This study aimed to compare weight changes in antiretroviral-naive people randomized to doravirine (DOR), efavirenz (EFV), or the protease inhibitors darunavir/ritonavir (DRV/r) and other antiretrovirals.
The three phase 2 or 3 trials were MK-1439A P007 (DOR versus EFV with TDF/emtricitabine [FTC]), DRIVE-FORWARD (DOR versus DRV/r with TDF/FTC or abacavir/3TC), and DRIVE-AHEAD (DOR/TDF/3TC versus EFV/TDF/FTC). The combined analysis included 855 people taking DOR, 383 taking DRV/r, and 472 taking EFV.
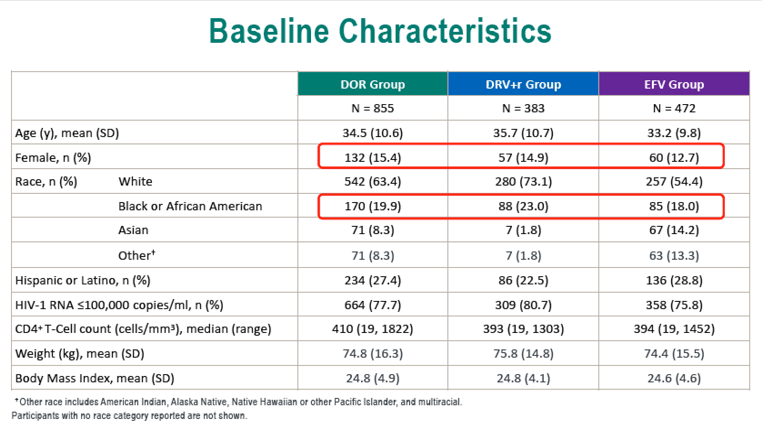
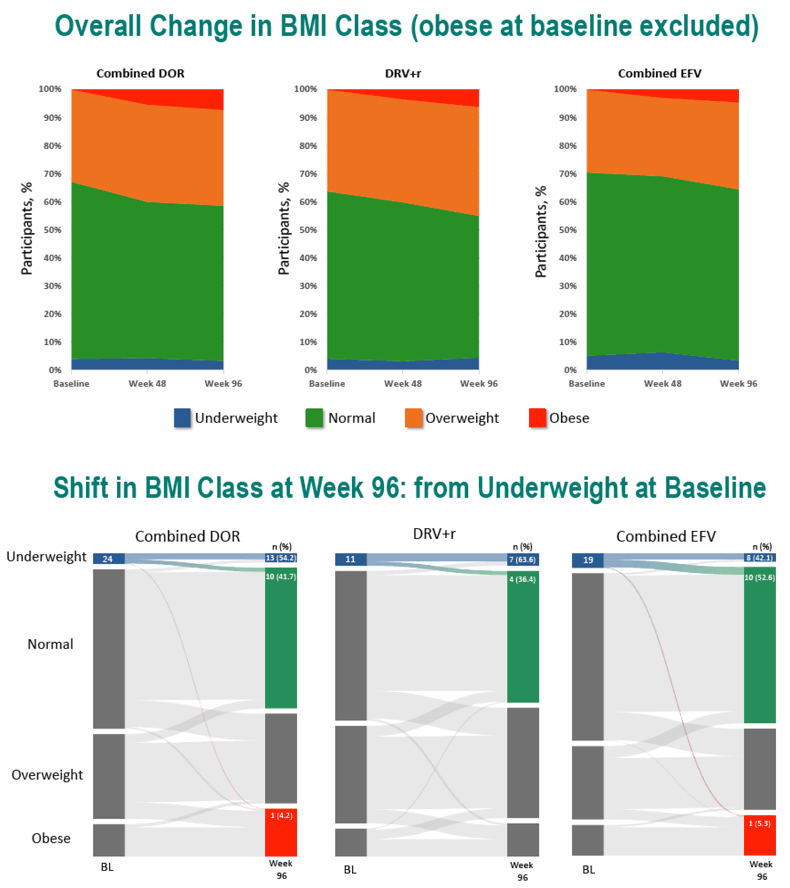
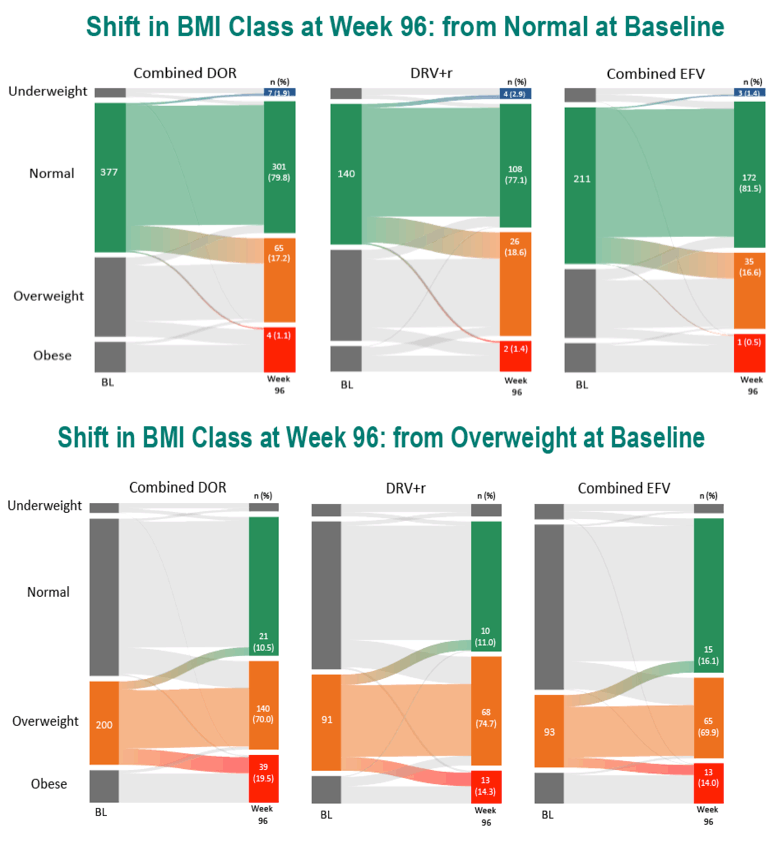
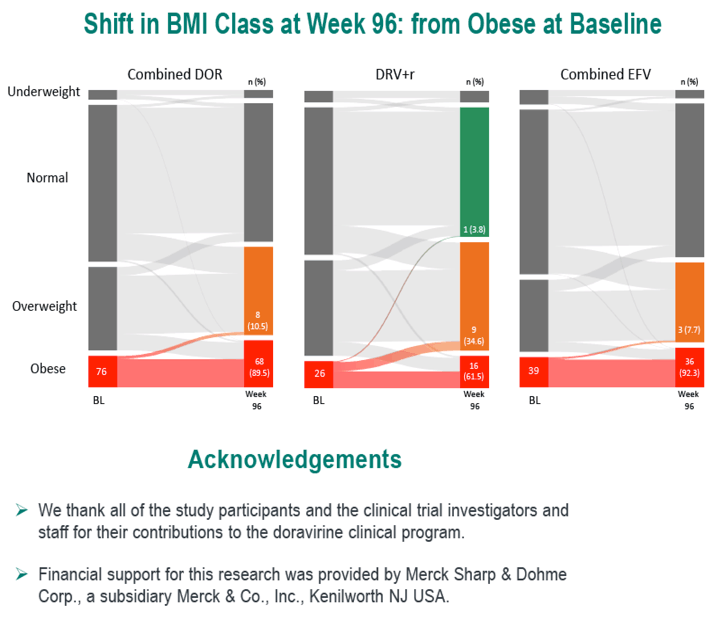
In the DOR, DRV/r, and EFV groups, age averaged 34.5, 35.7, and 33.2. Respective proportions of women were 15.4%, 14.9%, and 12.7%, of blacks 19.9%, 23.0%, and 18.0%, and of whites 63.4%, 73.1%, and 54.4%. When the trials began, weight averaged about 75 kg in all treatment groups and average body mass index (BMI) lay between 24.6 and 24.8 kg/m2--just below the overweight threshold.
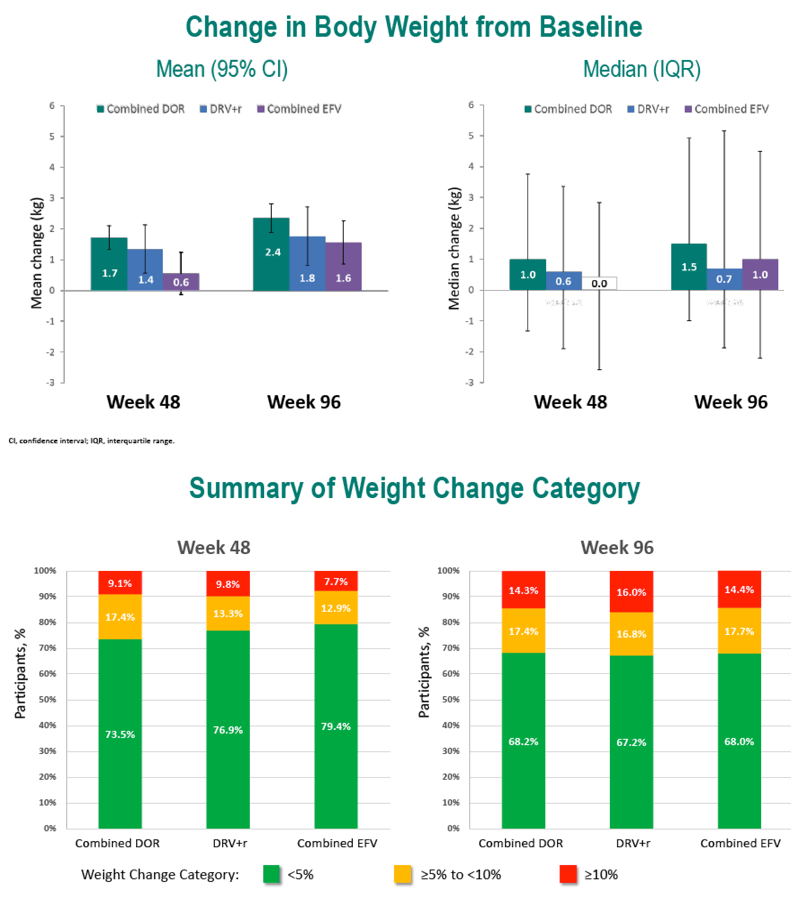
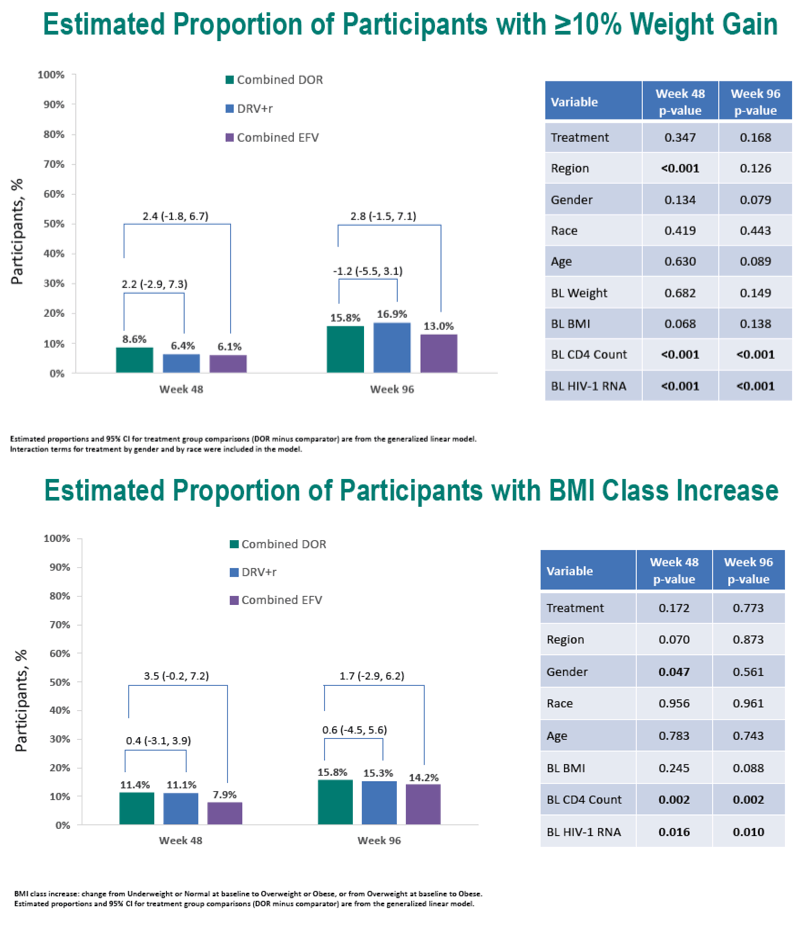
Through 96 weeks, the DOR group gained an average 2.4 kg (median 1.5 kg), the DRV/r group added an average 1.8 kg (median 0.7 kg), and the efavirenz group put on an average 1.6 kg (median 1.0 kg). At study weeks 48 and 96, estimated proportions of participants who gained 10% or more weight were 8.6% and 15.8% taking DOR, 6.4% and 16.9% taking DRV/r, and 6.1% and 13.0% taking EFV. In this analysis, assigned antiretroviral, age, gender, race, baseline weight, and baseline BMI were not associated with a 10% or greater weight gain at week 48 or week 96. Low baseline CD4 count and high baseline viral load were linked to both a 10% or greater weight gain and a jump to a higher BMI category.
At weeks 48 and 96, estimated proportions of participants who jumped to a higher BMI class were 11.4% and 15.8% with DOR, 11.1% and 15.3% with DRV/r, and 7.9% and 14.2% with EFV.
The researchers concluded that weight gains through 96 weeks were low in all three treatment arms and similar to average yearly upswings in adults with HIV (0.5 to 1 kg per year).
References
1. Orkin C, Elion R, Thompson M, et al. Effect of doravirine on body weight and body mass index in treatment naive adults with HIV-1. 17th European AIDS Conference, November 6-9, 2019, Basel. Abstract PS3/2.
2. McCann K, Moorhouse M, Sokhela S, et al. The ADVANCE clinical trial: changes from baseline to week 96 in DXA-assessed body composition in TAF/FTC+DTG compared to TDF/FTC+DTG, and TDF/FTC/EFV. 17th European AIDS Conference, November 6-9, 2019, Basel. Abstract 3/3.


|
| |
|
 |
 |
|
|