 |
 |
 |
| |
Maintenance DTG/3TC Controls HIV in People With Historical 3TC Resistance
"Dolutegravir and lamivudine for maintenance of HIV viral suppression in adults with and without historical resistance to lamivudine: 48-week results of a pilot clinical trial (ART-PRO)"
|
| |
| |
17th European AIDS Conference, November 6-9, 2019, Basel
Mark Mascolini
In a pilot trial of people with historical resistance to lamivudine (3TC), a dolutegravir (DTG)/3TC regimen maintained viral control for 48 weeks--as long as initial proviral DNA genotyping did not detect persistence of 3TC resistance mutations [1]. DTG/3TC also maintained viral control in people without historical resistance to 3TC.
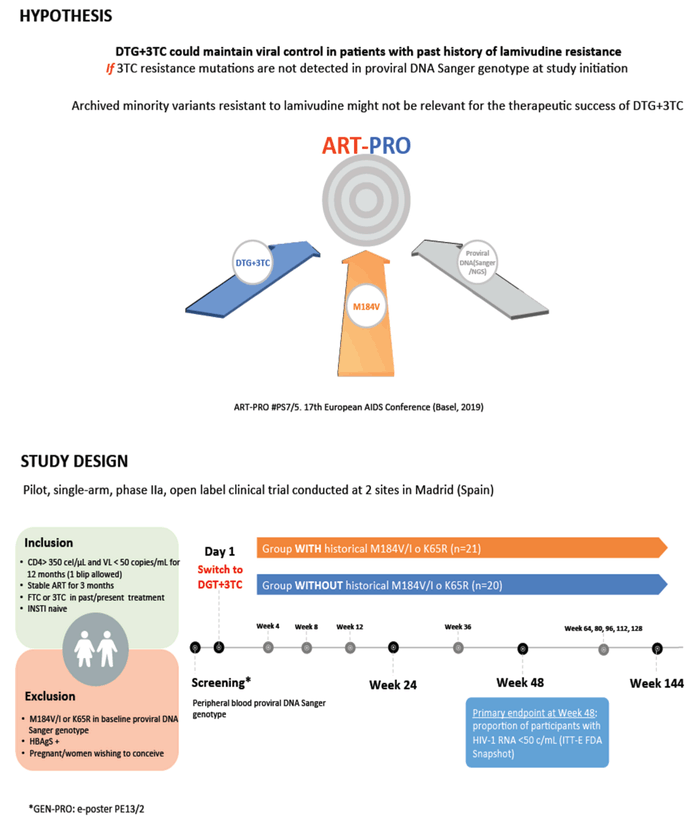
A tenet of HIV medicine is to avoid any antiretroviral in a person with evidence of prior mutations conferring resistance to that drug. Spanish researchers conducting the ART-PRO trial noted that 3TC may be an exception to this rule for several reasons: (1) it can retain antiviral activity in people with virologic failure and the hallmark 3TC mutation, M184V/I, (2) it adds antiviral activity in virologically suppressed people who had M184V in the past, (3) resistance mutations may not persist in a person's viral population or may persist as a minority variant in a defective form, and (4) the clinical relevance of antiretroviral-resistant minority variants in people with viral suppression remains uncertain.
These Madrid investigators hypothesized that DTG/3TC can maintain viral suppression in people with a history of 3TC resistance if proviral DNA Sanger genotyping does not detect 3TC resistance mutations before DTG/3TC begins. They suggested that archived minority variants resistant to 3TC "might not be relevant for the therapeutic success of DTG + 3TC."
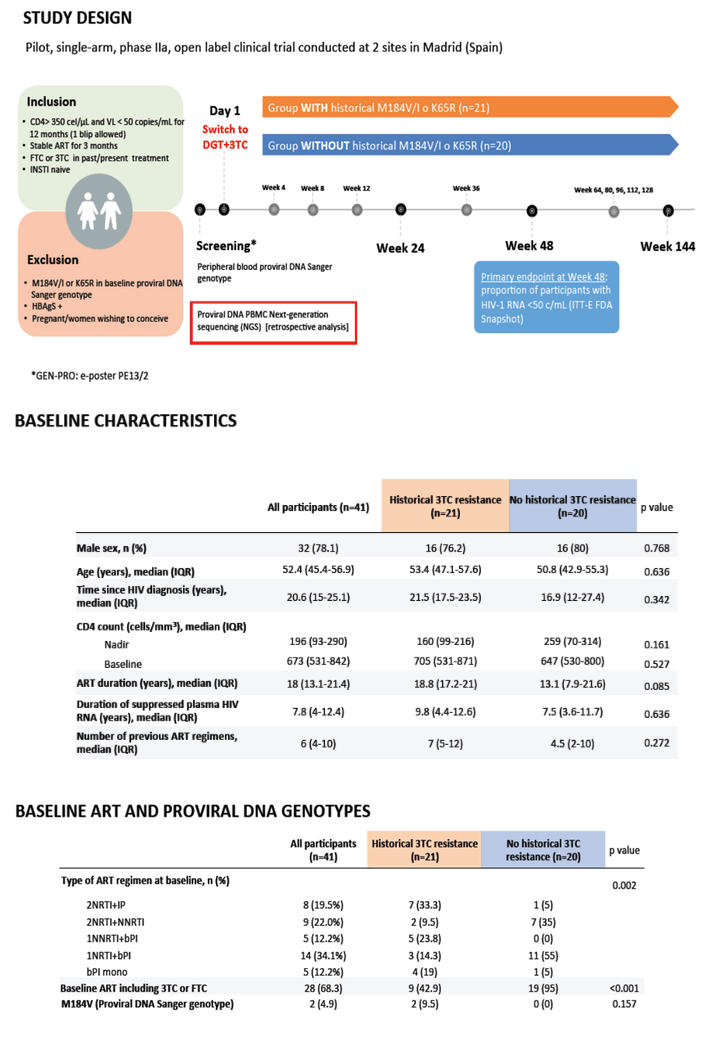
This single-arm, open-label, two-site study included people with a CD4 count above 350, a viral load below 50 copies for the past 12 months, stable antiretroviral therapy for the past 3 months, and 3TC or emtricitabine (FTC) in a past or current regimen. Prior treatment with an integrase inhibitor was not allowed. The trial excluded people with the M184V/I mutation or the tenofovir-related K65R mutation detected by proviral DNA Sanger genotyping. After screening, participants began DTG/3TC. The primary endpoint was the proportion of participants with a viral load below 50 copies at week 48 by an intention-to-treat-exposed FDA snapshot analysis.
While 21 participants had historical 3TC resistance, 20 did not. These two groups did not differ significantly in proportions of men (78% overall), age (median 52.4), time since HIV diagnosis (median 20.6 years), antiretroviral therapy duration (median 18.8 years in the historical 3TC resistance group and 13.1 years in the no-resistance group, P = 0.085), duration of viral control (median 7.8 years overall), or number of previous antiretroviral regimens (median 6). Nine of 21 people in the historical 3TC resistance group (43%) and 19 of 20 (95%) in the no-resistance group had 3TC or FTC in their baseline regimen (P < 0.001).
In the intention-to-treat population, 18 of 21 people with historical 3TC resistance and 20 of 20 without historical 3TC resistance had a week-48 viral load below 50 copies (85.7% versus 100%). The 3 participants in the historical 3TC resistance arm without a week-48 viral load below 50 copies included 1 person who withdrew because of an adverse event and 2 who withdrew because of protocol violations. In a per-protocol analysis, 18 of 19 people in the historical 3TC resistance group and 20 of 20 in the no-resistance group had a week-48 viral load below 50 copies.
No one in either study group had virologic failure. Ten participants (4 in the historical 3TC resistance group) had a transient viral rebound during the study; all regained viral control while continuing DTG/3TC. Retrospectively performed baseline proviral DNA next-generation sequencing identified several participants with 3TC resistance mutations, but those mutations had no impact on viral control at 48 weeks.
The Madrid team recorded 29 drug-related adverse events, 4 of which were severe but only 1 of which (insomnia) led to withdrawal from the study.
The researchers cautioned that findings in this small trial should be considered preliminary "and need to be confirmed in a fully powered study." Until then, "this strategy should be considered experimental."
Reference
1. De Miguel Buckley R, Rial Crestelo D, Dominguez-Dominguez L, et al. Dolutegravir and lamivudine for maintenance of HIV viral suppression in adults with and without historical resistance to lamivudine: 48-week results of a pilot clinical trial (ART-PRO). 17th European AIDS Conference, November 6-9, 2019, Basel. Abstract PS7/5.


|
| |
|
 |
 |
|
|