 |
 |
 |
| |
Retrospective analysis of co-medication patterns among patients treated for HIV, and potential interactions with antiviral treatment, in Norway during 2012-2018 using the Norwegian population-based prescription database
|
| |
| |
17th European AIDS Conference, November 6-9, 2019, Basel
Reported by Jules Levin
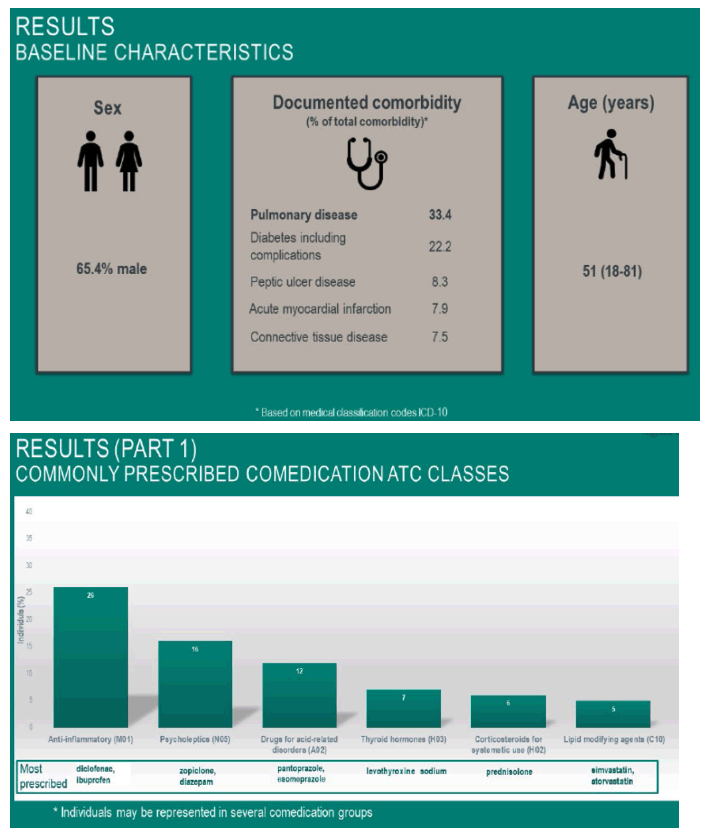
average age 51 (18-81): 33% pulmonary disease, diabetes & complications 22%, peptic ulcer disease 8%, acute myocardial infarction 8%, connective tissue disease 7.5%. "The main change in Norway toddies not viral control but management of comorbidities in PLWH" !!!!! - from Jules
L. Heggelund1, R. Singh2, S. Aballi3, K. Hagen4, C.M. Rosseland2, Y. Mikkelsen5, J.K. Damås6 1Drammen Hospital, Drammen, Norway, 2MSD AS, Drammen, Norway, 3Kalnes Hospital, Tønsberg, Norway, 4MSD, Stockholm, Sweden, 5LINK Medical AS, Oslo, Norway, 6St. Olav Hospital, Trondheim, Norway
Program abstract
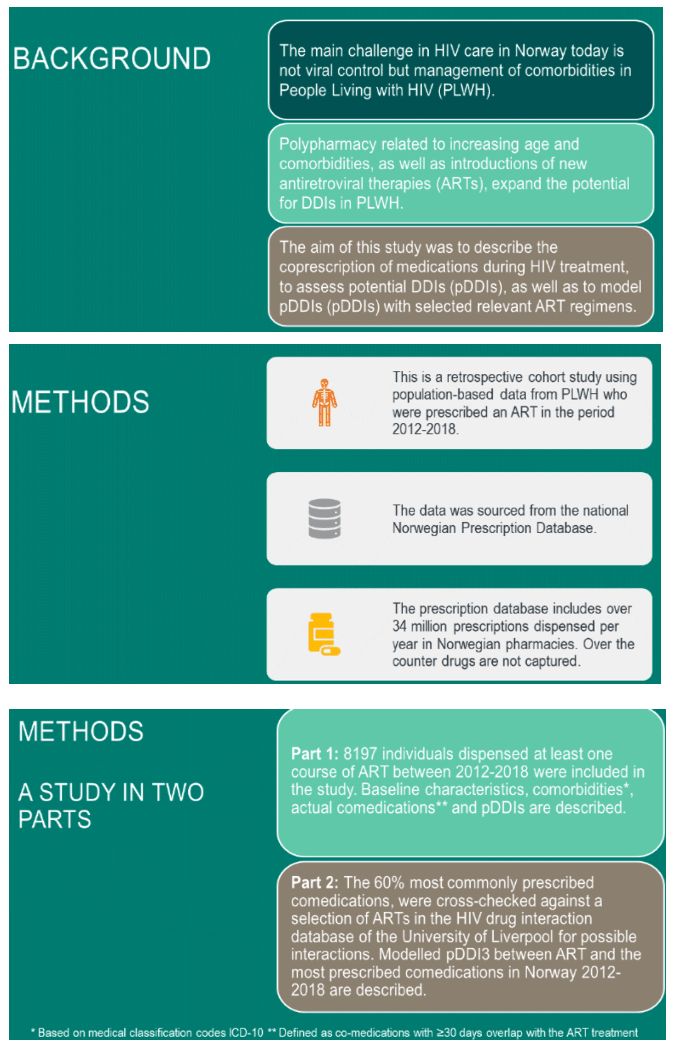
Background: The potential for drug-drug interactions (DDIs) is an important consideration for HIV-infected individuals with concomitant medications. The aim of this study was to describe the co-administration of medication during HIV treatment, and to assess potential DDIs (pDDIs) with currently approved antiretroviral treatments.
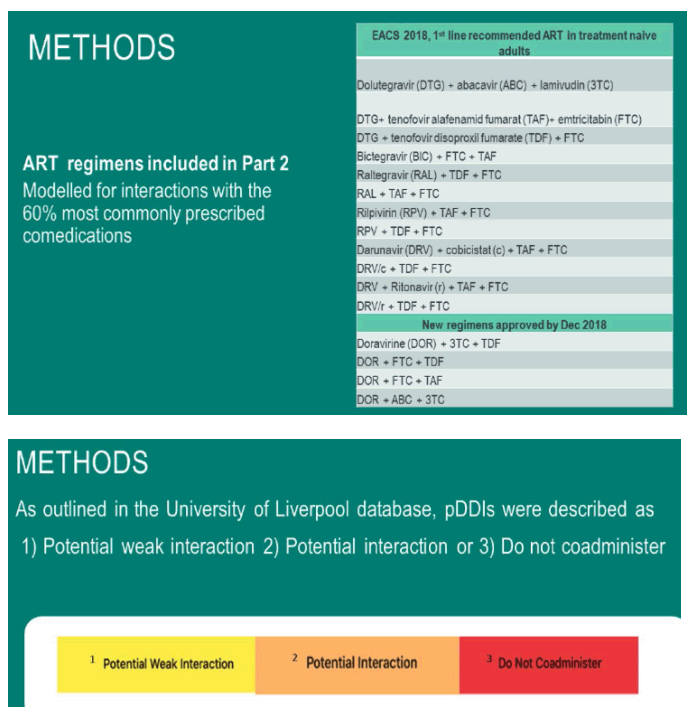
Method: This is a retrospective cohort study using population-based data from patients prescribed antiretrovirals in the period 2012-2018. Data was sourced from the Norwegian Prescription Database. 60% of the most commonly prescribed comedications, , were cross-checked against the antiretroviral medicines in the HIV drug interaction database of the University of Liverpool. pDDIs are described as either 1) potential weak interaction, 2) potential interaction and 3) do not co-administer.
Results: 8197 patients dispensed at least one HIV treatment course were included. Median age was 51 years (< 18-81) and 65.4% were male.
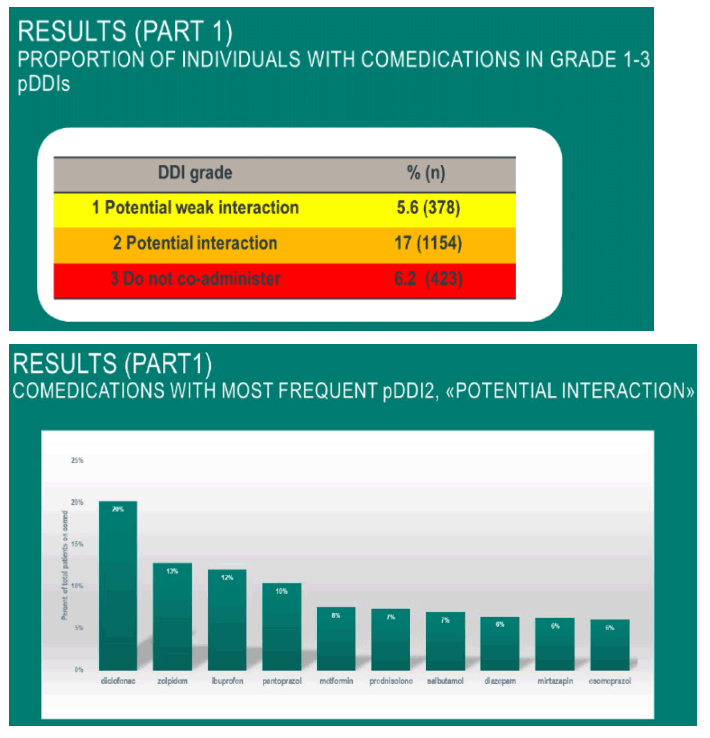
82.7% of HIV-treated patients received comedications, with minimum 30 days of overlapping treatments.
The most commonly prescribed comedication ATC classes were anti-inflammatory and antirheumatic products in 26%, psycholeptics in 16% and drugs for acid-related disorders in 12% of total comedicated patients.
Among patients with comedication, 5.6 % (378 patients), 17% (1154 patients) and 6.2% (423 patients) were prescribed at least one comedication with a pDDI in the grade 1, 2 and 3 categories, respectively.
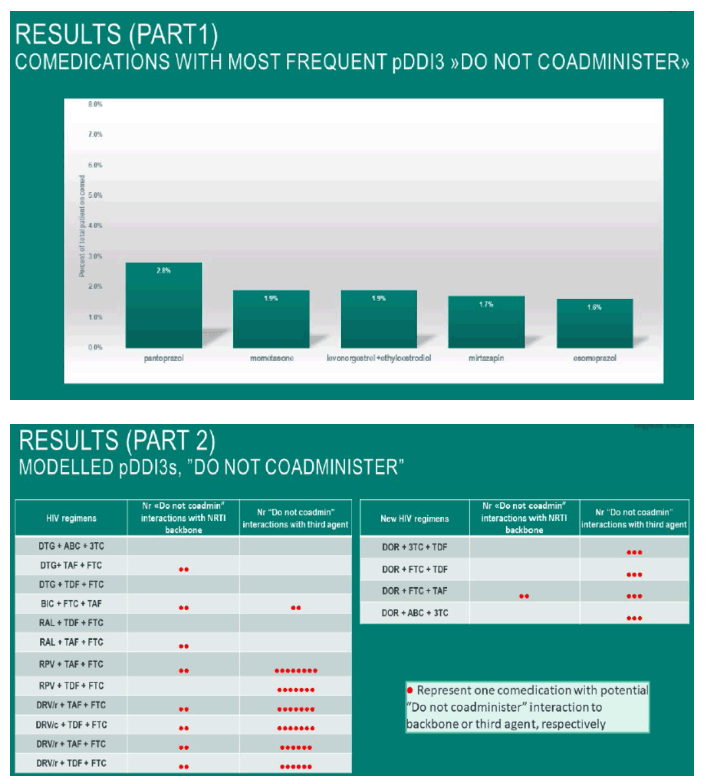
Most grade 3 pDDIs were seen in comedications with pantoprazol, mometasone and levonorgestrel+ethyloestradiol.
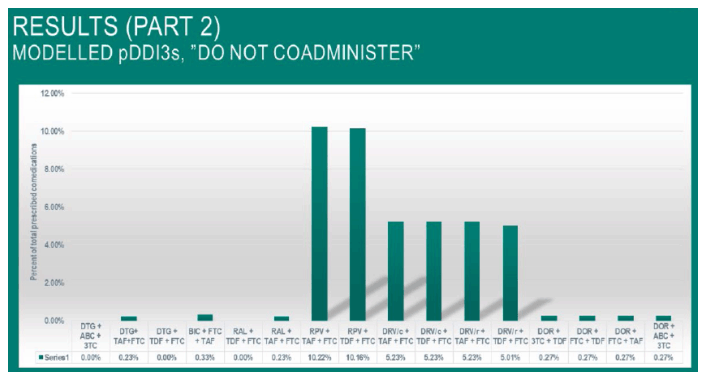
When modelling the 60% most prescribed comedications and cross referencing with the EACS recommended regimens with an integrase-inhibitor as the third agent, the percentage of modelled pDDIs ranged from 0.0- 0.33% grade 3pDDI in dolutegravir, raltegravir and bictegravir -containing regimens.
Conclusion: Data from a large population-based prescription data base showed that co-administration of other drugs are frequent during antiretroviral treatment, and the potential for DDIs needs to be considered to optimize HIV care.
|
| |
|
 |
 |
|
|