 |
 |
 |
| |
Retreatment with sofosbuvir/velpatasvir/voxilaprevir in patients with chronic hepatitis C virus infection and prior DAA failure - an analysis from the German Hepatitis C-Registry (DHC-R)
|
| |
| |
Reported by Jules Levin
EASL 2019 April 10-14 Vienna
Johannes Vermehren1*Albrecht Stoehr2, Julian Schulze zur Wiesch3, Hartwig Klinker4, Markus Cornberg5, Maria-Christina Jung6, Karl-Georg Simon7, Yvonne Serfert8, Michael P. Manns5, Heiner Wedemeyer5,8,9, Christoph Sarrazin1,10, German Hepatitis C-Registry8
1Goethe University Hospital, Medical Clinic 1, Frankfurt, Germany; 2ifi-Institute for Interdisciplinary Medicine, Hamburg, Germany; 3University Medical Center Hamburg-Eppendorf (UKE), Hamburg, Germany; 4University Hospital Würzburg, Würzburg, Germany; 5Hannover Medical School, Hannover, Germany; 6Leberzentrum München, Germany; 7MVZ Dres. Eisenbach, Simon, Schwarz GbR, Leverkusen, Germany; 8Leberstiftungs-GmbH Deutschland, Hannover, Germany; 9Essen University Hospital, University of Duisburg-Essen, Essen, Germany; 10St. Josefs-Hospital, Medical Clinic 2, Wiesbaden, Germany
EASL Press Release. I reported earlier this morning the TRIO data on SOF/VEL/VOX retreatment mentioned in this release.
'This important real-world experience from Germany and the USA showed that almost all patients with chronic hepatitis C - Including DAA treatment failures - can finally be cured," said Professor Markus Cornberg from Hannover Medical School in Germany, a member of EASL's governing board.
'Real-world' studies conducted in Germany and the USA have confirmed the effectiveness and tolerability of sofosbuvir/velpatasvir/voxilaprevir (SOF/VEL/VOX) when used to treat individuals with chronic hepatitis C (HCV) infection who had previously failed direct-acting antivirals (DAAs). The two studies presented today at The International Liver Congress™ 2019 in Vienna, Austria, reported sustained virological response rates (SVR) of 93-100%, confirming the effectiveness of this treatment in clinical practice.
DAAs have transformed HCV therapy and made virological cure possible in most patients.1 Combination regimens of DAAs achieve SVR rates in excess of 90%, regardless of HCV genotype (GT), disease stage, or treatment history.2 While DAAs rarely fail to achieve viral clearance, up to 10% of patients will experience virological relapse/failure,3 with HCV RNA reappearing a few weeks after completing therapy. In the past, treatment options for these patients were limited.4,5 The once-daily, all-oral combination tablet containing SOF/VEL/VOX, was approved in Europe in July 20176 for patients infected with any genotype who have previously failed therapy with DAAs.1
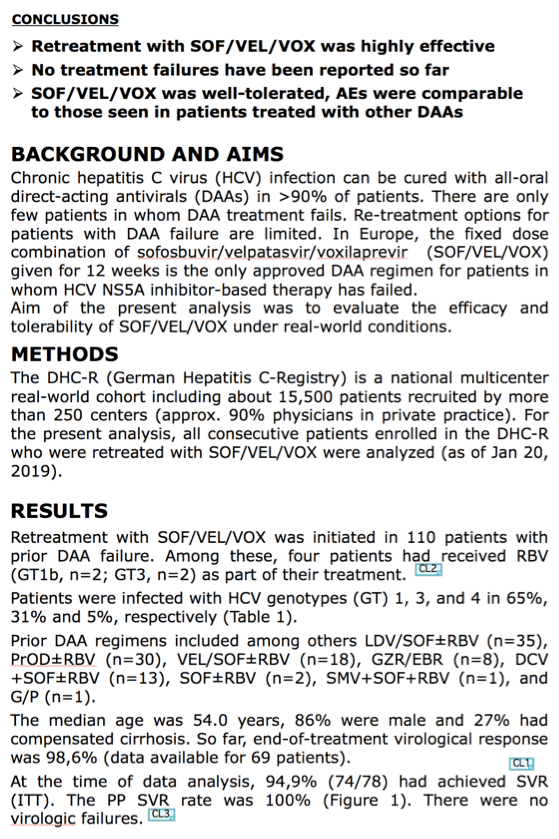
In the first study presented today, a German research group evaluated all consecutive patients enrolled into the German Hepatitis C Registry (DHC-R) who were retreated with SOF/VEL/VOX ± ribavirin (RBV) due to virological failure up to February 2019 (N=110; HCV GT1 [71%], GT3 [34%], and GT4 [5%]; median age 54 years; 86% male; 27% with cirrhosis). Prior DAA regimens included paritaprevir/ritonavir/ombitasvir ± dasabuvir ± RBV (PrO ± D ± RBV [n=30]), ledipasvir/sofosbuvir ± RBV (LDV/SOF ± RBV [n=35]), SOF/velpatasvir ± RBV (SOF/VEL ± RBV [n=18]), daclatasvir + SOF ± RBV (DCV + SOF ± RBV [n=13]), elbasvir/grazoprevir (EBR/GZR [n=8]), SOF + RBV (n=2), and simeprevir + SOF + RBV (n=1). Four patients had received SOF/VEL/VOX + RBV (HCV GT1b [n=2], GT3a [n=2]).
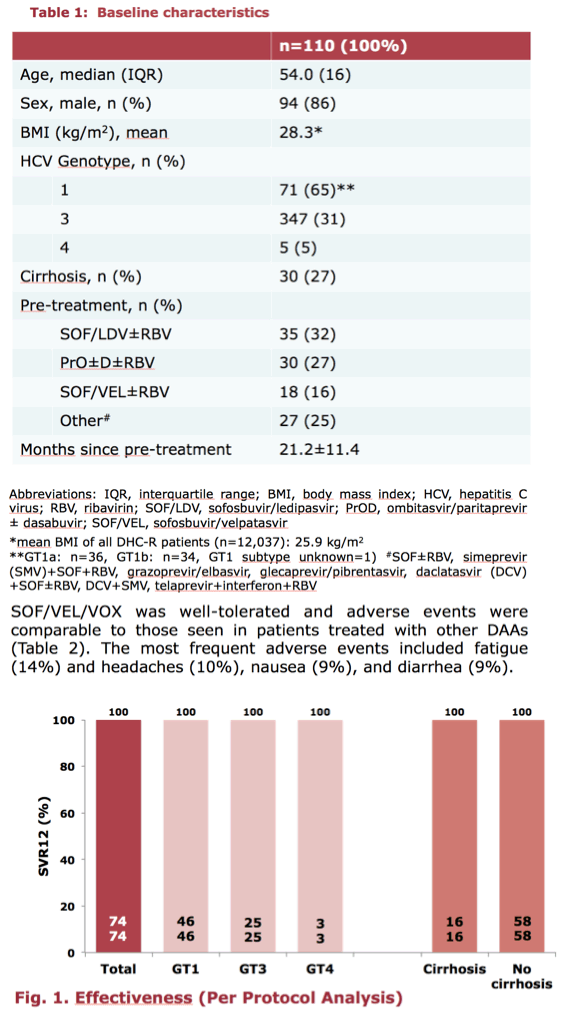
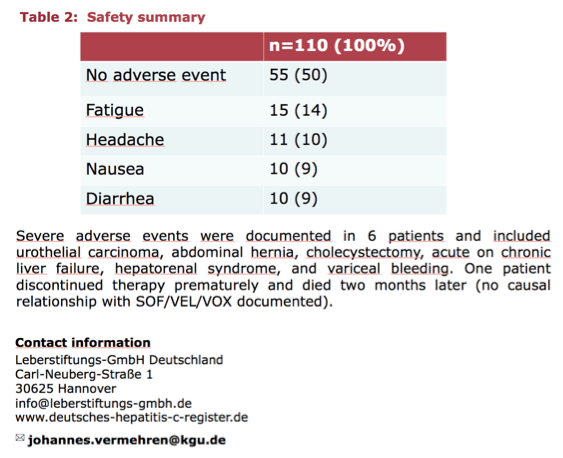
According to investigator Dr Johannes Vermehren from the Goethe University Hospital in Frankfurt, Germany, with SVR data available from 74 patients as of February 2019, sustained virologic response was 100%. SOF/VEL/VOX was well tolerated, with fatigue (14%) and headaches (10%) the most frequently reported adverse events (AEs). No severe AEs were attributed to SOF/VEL/VOX treatment.
In the USA, data from 196 patients treated with SOF/VEL/VOX between July 2017 and April 2018 were collected from a health management program provider (Trio Health) and analysed. Duration of treatment was 12 weeks for all but one patient, who received treatment for >12 weeks. Seven patients (4%) also received RBV off-label. Most patients were treatment experienced (173/196; 88%) while 21 patients (11%) were treatment naïve. Treatment status for two patients was unavailable. The most frequently-used prior therapies were: LDV/SOF ± RBV (n=92), SOF/VEL ± RBV (n=20), EBR/GZR ± RBV (n=19), other SOF-based regimens (n=17), and PrOD (n=11). The SVR rates at 12 weeks after therapy in the per-protocol and intent-to-treat groups were reported to be 98% (183/186) and 93% (183/196), respectively. Of the 3 patients that did not achieve SVR12 in the per-protocol group, 2/3 were white males, cirrhotic (F4), and had prior regimens of SOF/VEL and LDV/SOF ± RBV while 1/3 was a black female with moderate fibrosis (F2); her prior regimen was not specified. One patient had a baseline viral load between 800K-6MM and the other 2 patients had baseline viral loads >6MM. The insurance types were: 1 (commercial), 1 (Medicare), and 1 (not specified); all 3 patients were GT1.
'We are now seeing real-world evidence that SOF/VEL/VOX is highly effective when used in clinical practice to treat patients who have failed previous DAA therapy,' concluded Dr Bruce Bacon from Saint Louis University School of Medicine, St Louis, USA.
'This important real-world experience from Germany and the USA showed that almost all patients with chronic hepatitis C - Including DAA treatment failures - can finally be cured," said Professor Markus Cornberg from Hannover Medical School in Germany, a member of EASL's governing board.
---------------------------------
|
| |
|
 |
 |
|
|