 |
 |
 |
| |
Uptake of testing and treatment for HCV among PWID in Australia:
The ETHOS Engage Study - "59% HCV treatment uptake"
|
| |
| |
"Australia has had unrestricted government subsidised direct-acting antiviral therapy for HCV since March 2016. "
Conclusion: The DAA era in Australia has produced high treatment uptake and lowered HCV viraemia among PWID attending drug treatment and needle syringe programs. To reach elimination targets, subgroups of PWID may require additional support to encourage further screening and engagement with HCV care.
"......HCV treatment uptake highly encouraging (59% overall had received treatment).......we have achieved high treatment uptake across most of the population (even in people with >daily injecting the treatment uptake is impressive)..... the key point is that we have reduced the viremic prevalence to 27%, which is half-way there......We will be conducting a second wave in 2019-2020 which will include a test and treat strategy, so hopefully we can continue to drive the HCV RNA prevalence down."
from Jules: There is no federal funded program to try to treat all PWIDs
Reported by Jules Levin
EASL 2019 April 10-4 Vienna
Heather Valerio
Maryam Alavi, David Silk, Carla Treloar, Andrew Milat, Adrian Dunlop,
Jo Holden, Charles Henderson, Phillip Read, Janaki Amin, Louisa Degenhardt,
Gregory Dore, Jason Grebely

program abstract
Uptake of testing, linkage to care, and treatment for hepatitis C infection among people who
inject drugs in Australia: The ETHOS Engage study
Heather Valerio1, Maryam Alavi1, David Silk1, Carla Treloar2, Andrew Milat 3, Adrian Dunlop4 5, Jo
Holden 6, Charles Henderson 7, Janaki Amin8, Phillip Read9, Louisa Degenhardt10, Gregory Dore1,
Jason Grebely1
1The Kirby Institute, UNSW Sydney, Sydney, Australia; 2Centre for Social Research in Health, UNSW
Sydney, Sydney, Australia; 3Centre for Epidemiology and Evidence, NSW Health, Sydney, Australia;
4Hepatitis NSW, Sydney, Australia; 5Drug and Alcohol Clinical Services, Hunter New England Local
Health District, Sydney, Australia; 6Population Health Strategy and Performance, NSW Health,
Sydney, Australia; 7NSW Users and AIDS Association, Sydney, ; 8Macquarie University, Sydney,
Australia; 9Kirketon Road Centre, Sydney, Australia; 10National Drug and Alcohol Research Centre,
UNSW Sydney, Sydney, Australia
Background and aims: People who inject drugs (PWID) are at high risk of hepatitis C virus (HCV)
infection but have poor access to HCV treatment in most settings. Unrestricted direct-acting antiviral
(DAA) therapy has been available in Australia since March 2016. Our objective was to evaluate
burden of HCV and the extent of, and factors associated with, engagement with the HCV cascade of
care among PWID in an era of unrestricted DAA therapy access.
Method: ETHOS Engage is an observational cohort study collecting demographic, behavioural and
clinical data among PWID attending drug treatment clinics and needle and syringe programs in
Australia. All PWID underwent point-of-care (POC) HCV RNA testing via Xpert® HCV Viral Load
Finger-Stick assay. Multivariate logistic regression models were used to identify demographic and
behavioural factors associated with treatment uptake.
Results: Between May and November 2018, 507 PWID were enrolled. Overall, 70% had injected
drugs in the last month, and 70% were currently receiving opioid substitution therapy (OST). Of all
enrolled, 73% (n = 370) were ever HCV-infected (Ab positive), and 58% (n = 296) were ever chronic
HCV-infected (RNA positive or prior treatment). Among those Ab positive (n = 370), 76% had
previously been tested for HCV RNA. Among those with evidence of current or past chronic HCV (n =
296), 86% had ever been linked to care and 68% had ever received treatment for HCV. Uptake of
HCV therapy was high across sub-populations, including those with current and no OST (71%, 59%)
recent injecting (last month) (70%), and heroin (68%), other opioid (57%) and amphetamine (70%)
injecting in the last month. In adjusted analysis, no factors were associated with treatment uptake.
Among those with a POC HCV RNA result at enrolment, 26% had current HCV infection (HCV
RNA+ve), 33% had treatment-induced clearance, 17% spontaneous clearance, and 23% were
uninfected (HCV Ab-ve) (Figure). The proportion with current HCV infection was similar across
demographic and behavioural sub-populations.
Conclusion: The DAA era in Australia has produced high treatment uptake and lowered HCV
viraemia among PWID attending drug treatment and needle syringe programs. To reach elimination
targets, subgroups of PWID may require additional support to encourage further screening and
engagement with HCV care.


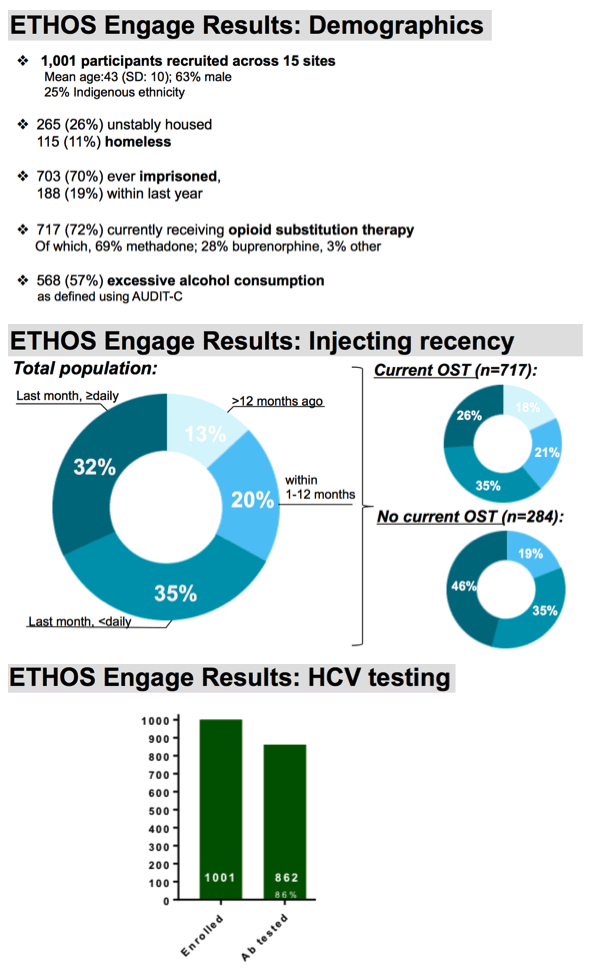
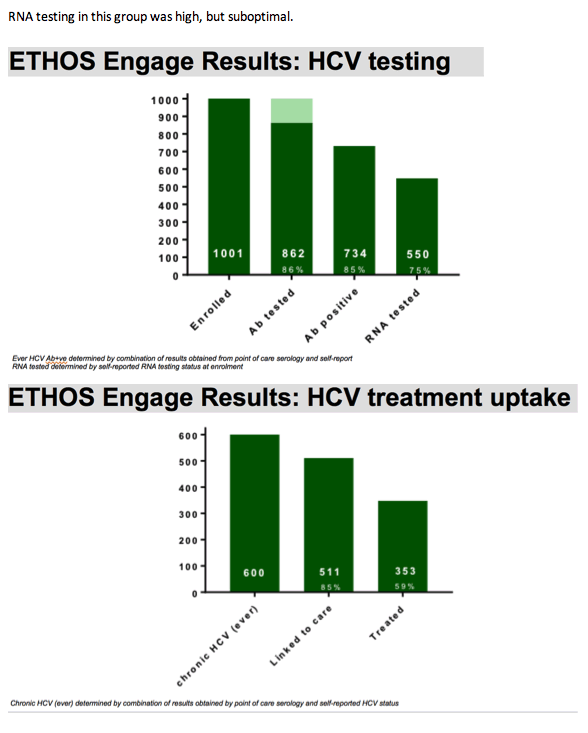
Among our 1001 enrolled participants, the vast majority, 86%, had ever been screened for hepatitis c and had ever received an antibody test. Antibody positivity was determined by a combination of results obtained by POC serology and self-reported HCV status, where all participants who tested positive upon point of care, who indicated ever receiving treatment, or had indicated ever being infected with HCV were considered ever Ab positive. This comprised 73% of our enrolled participants and is consistent with results obtained with the most previous Australian Needle and Syringe survey. Among these, at baseline, 75% had indicated ever being tested for HCV RNA.
RNA testing in this group was high, but suboptimal.
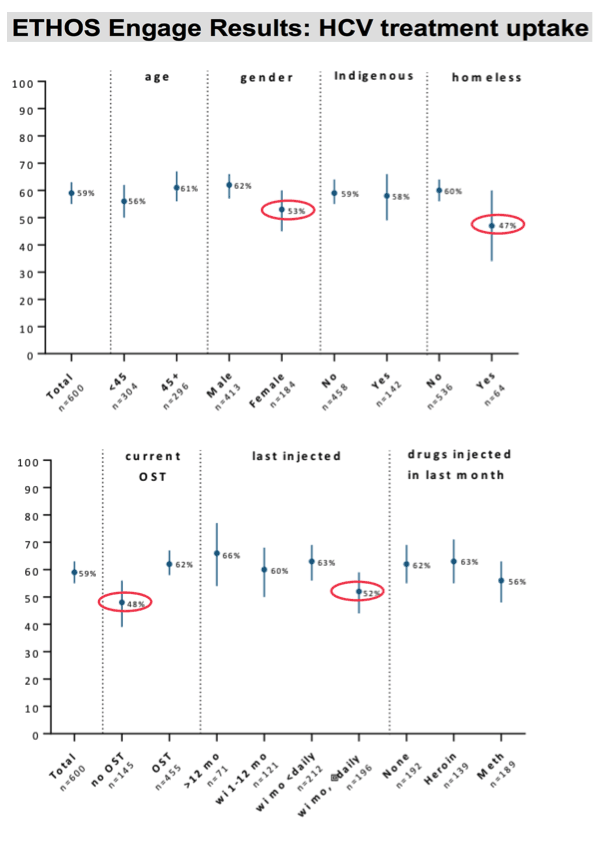
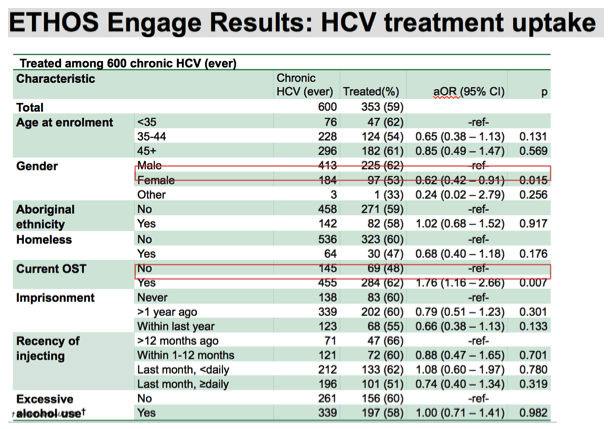
After adjusting for all covariates listed in the first column of the slide ,male gender was significantly associated with treatment uptake , as well as OST status, where both males and those currently receiving OST were more likely to initiate treatment than females or non OST recipients, respectively.
There was no significant associations observed among other covariates, including recent injectors, homelessness, or among our age groups.
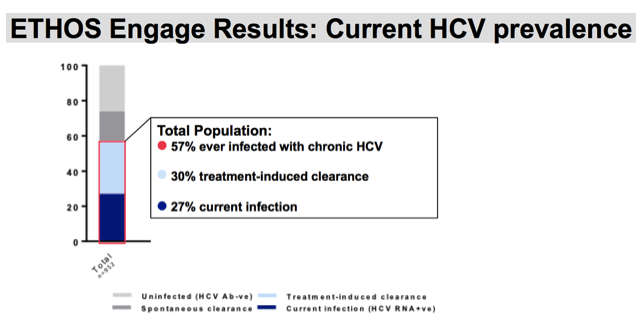
5% of our recruited population did not have a valid POC result and have been excluded from our prevalence reports. Our HCV prevalence groups have been identified using an algorithm of point of care result and self-report. Blue bars indicate our population of participants who have ever been infected with chronic HCV. Light blue bars represent those who have indicated receiving HCV treatment, ever, and who have a POC result as negative. . Current HCV infection in noted in the navy blue bars.
Among our entire recruited population with a valid POC result ,57% had ever had hepatitis C viremia; 30% had treatment-induced clearance; and 27% were currently infected.
We can use this as a tool to determine how we are tracking toward elimination, and what sub-populations may need additional support to facilitate increased treatment uptake and a subsequent reduction in HCV viremia
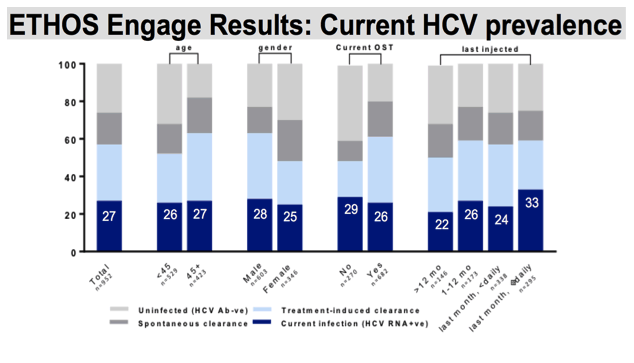
When we stratify prevalence, the proportion of hepatitis C infection was similar across demographic and behavioural sub-populations. Lowest ever HCV viremia was noted among females and those not on OST, with 48% ever infected. 25% of our female participants were currently infected, and 29% of those not on OST had current HCV infection. This was the second highest proportion of current infection, behind those who indicated injecting daily or more within the last month, 33% of which had current HCV viremia.
|
| |
|
 |
 |
|
|