 |
 |
 |
| |
Reinfection following successful HCV DAA therapy
among people with recent injecting drug use
|
| |
| |
Reported by Jules Levin
EASL 2019 April 10-14 Vienna

program abstractbr clear="all" />
Background and aims: HCV direct acting antiviral (DAA) therapy is effective in people who inject drugs however, little is known about HCV reinfection following DAA therapy among people who have recently injected drugs and/or people receiving opioid substitution therapy (OST). The aim of this study was to evaluate HCV reinfection following successful DAA therapy among people with recent injecting drug use.
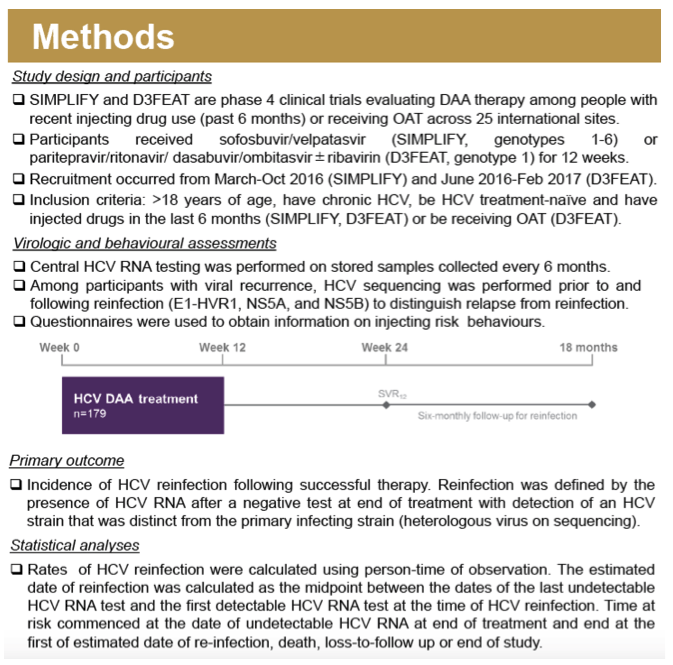
Method: SIMPLIFY and D3FEAT are phase IV clinical trials of DAA therapy among people with recent injecting drug use (IDU; last six months) or those receiving OST, through a network of 25 international sites (SIMPLIFY: sofosbuvir/velpatasvir for 12 weeks in people with recent injecting; D3FEAT: paritaprevir/ritonavir/dasabuvir/ombitasvir ± ribavirin for 12 weeks in people with recent injecting or receiving OST). This analysis assessed HCV recurrence from end of treatment response (ETR) through 24 weeks post-ETR (SVR24).
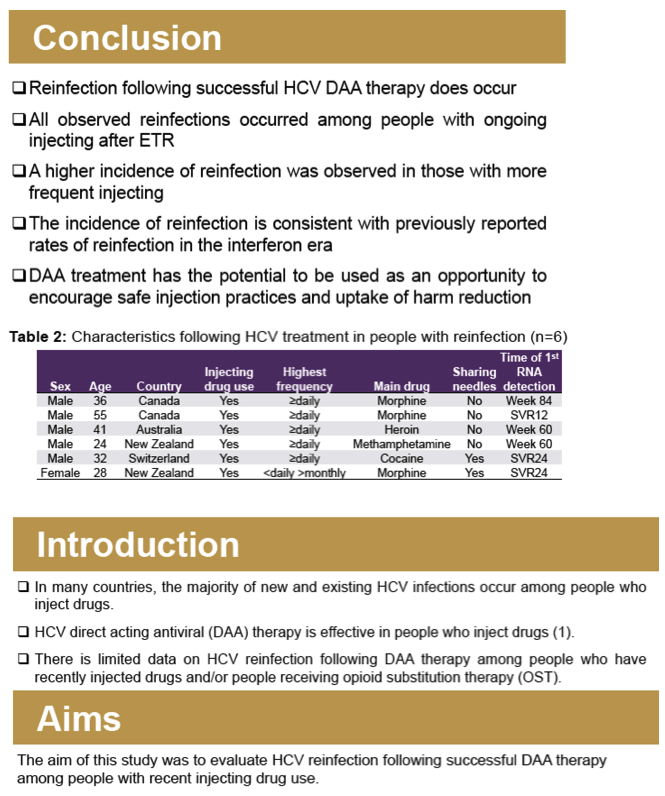
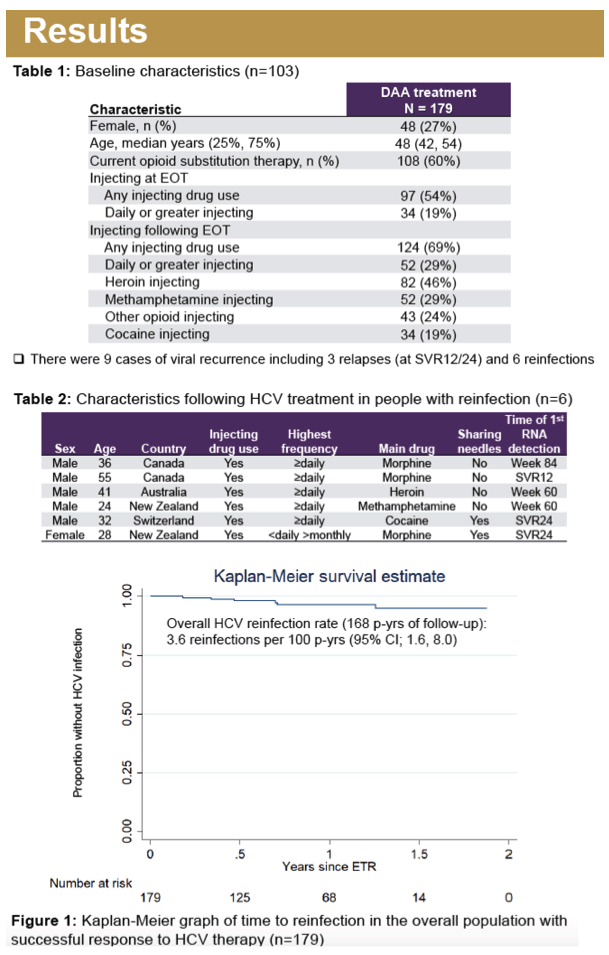
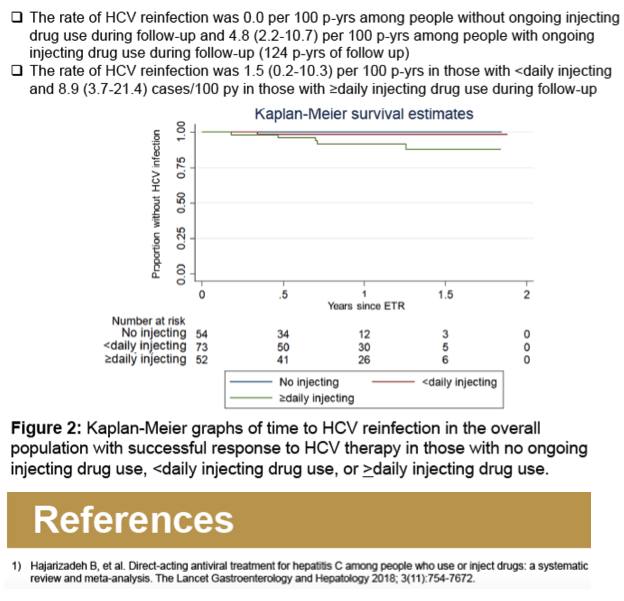
Results: Overall, 179 participants (72% male, median age 48 years) had an ETR and at least one subsequent follow-up visit in SIMPLIFY (n = 97) and D3FEAT (n = 82). At treatment initiation, 80% (n= 144) reported IDU in the past 6 months, 54% (n = 97) reported IDU in the past month, and 60% (n =108) were receiving OST. IDU between ETR and follow-up was reported in 70% (n = 125). HCV recurrence was observed in nine participants including three cases of HCV relapse and six cases of reinfection. Over 168 person-years (py) of follow-up, the incidence of reinfection was 3.6/100 py (95% CI 1.6-7.9). There were no cases of reinfection among those who did not report ongoing injecting drug use after ETR. The incidence of reinfection in those with ongoing injecting after ETR (124 py of followup) was 4.8/100 py (95% CI 2.2-10.7/100 py).
Conclusion: HCV reinfection can occur following HCV DAA therapy among people with ongoing injecting drug use following DAA therapy, with higher rates of HCV reinfection observed among people with ongoing injecting drug use following therapy.
|
| |
|
 |
 |
|
|