| |
Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial
|
| |
| |
Download the PDF here
Download the PDF here
Lancet HIV May 5 2019 - Hans-Jürgen Stellbrink, José R Arribas, Jeffrey L Stephens, Helmut Albrecht, Paul E Sax, Franco Maggiolo, Catherine Creticos, Claudia T Martorell,
Xuelian Wei, Rima Acosta, Sean E Collins, Diana Brainard, Hal Martin
Findings
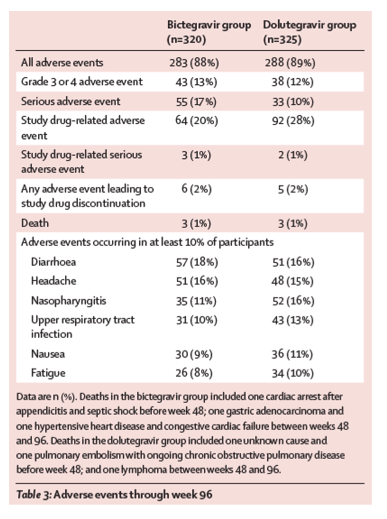
Between Nov 13, 2015, and July 14, 2016, we screened 742 individuals, of whom 657 were enrolled. 327 participants were assigned to the bictegravir group and 330 to the dolutegravir group. Of these, 320 in the bictegravir group and 325 in the dolutegravir group received at least one dose of study drug. At week 96, HIV-1 RNA less than 50 copies per mL was achieved by 269 (84%) of 320 participants in the bictegravir group and 281 (86%) of 325 in the dolutegravir group (difference -2⋅3%, 95% CI -7⋅9 to 3⋅2), demonstrating non-inferiority of the bictegravir regimen compared with the dolutegravir regimen. Both treatments continued to be well tolerated through 96 weeks; 283 (88%) of 320 participants in the bictegravir group and 288 (89%) of 325 in the dolutegravir group had any adverse event and 55 (17%), and 33 (10%) had any serious adverse event. The most common adverse events were diarrhoea (57 [18%] of 320 in the bictegravir group vs 51 [16%] of 325 in the dolutegravir group) and headache (51 [16%] of 320 vs 48 [15%] of 325). Deaths were reported for three (1%) individuals in each group (one cardiac arrest, one gastric adenocarcinoma, and one hypertensive heart disease and congestive cardiac failure in the bictegravir group and one unknown causes, one pulmonary embolism, and one lymphoma in the dolutegravir group); none were considered to be treatment related. Adverse events led to discontinuation in six (2%) participants in the bictegravir group and five (2%) in the dolutegravir group; one of these events in the bictegravir group versus four in the dolutegravir group occurred between weeks 48 and 96. Study drug-related adverse events were reported for 64 (20%) participants in the bictegravir group and 92 (28%) in the dolutegravir group.
Interpretation
These week 96 data support bictegravir, emtricitabine, and tenofovir alafenamide as a safe, well tolerated, and durable treatment for people living with chronic HIV.
--------------------------
Nine women had eleven confirmed pregnancies, five women with seven confirmed pregnancies in the bictegravir group and four women with four confirmed pregnancies in the dolutegravir group. Study drugs were interrupted or discontinued by the investigator when each pregnancy was confirmed. In the bictegravir group, the pregnancies resulted in an elective abortion in one woman, spontaneous abortion in three women, and uncomplicated term delivery in three women. In the dolutegravir group, the pregnancies resulted in elective abortion in one woman and uncomplicated term deliveries in three women.
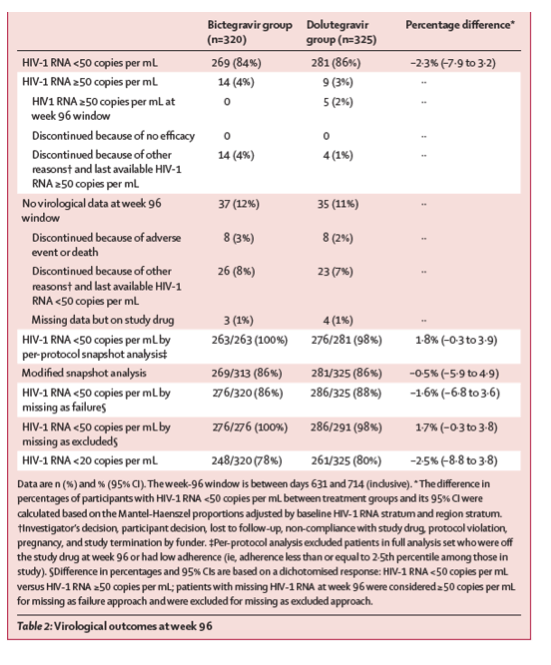
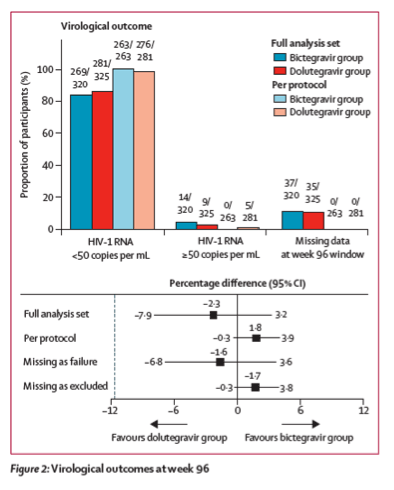
269 (84%) of 320 participants in the bictegravir group and 281 (86%) of 325 in the dolutegravir group had plasma HIV-1 RNA less than 50 copies per mL at week 96 (difference -2⋅3%; 95% CI -7⋅9 to 3⋅2; table 2, figure 2); therefore the bictegravir regimen was non-inferior to the dolutegravir regimen in the US FDA snapshot analysis. For the prespecified per-protocol analysis, 263 (100%) of 263 participants in the bictegravir group and 276 (98%) of 281 in the dolutegravir group had HIV-1 RNA less than 50 copies per mL (difference 1⋅8%, 95% CI -0⋅3 to 3⋅9). 14 (4%) participants in the bictegravir group and four (1%) in the dolutegravir group discontinued study drug because of reasons not related to study treatment and had last available HIV-1 RNA measurements of at least 50 copies per mL. 10
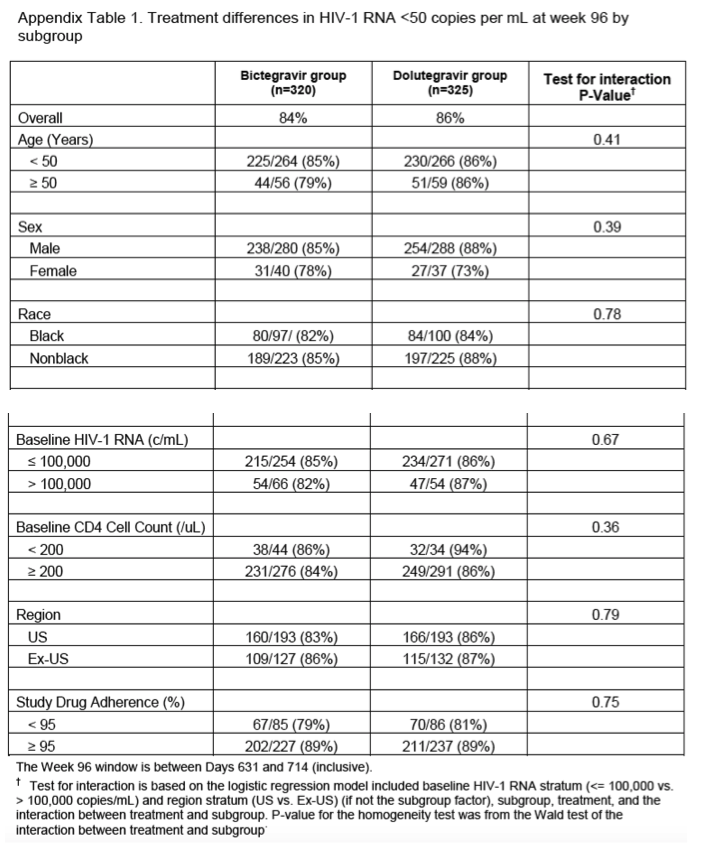
Seven of these participants from the bictegravir group did not have any HIV-1 RNA data after baseline. As such, their only available HIV-1 RNA, which was used to assess efficacy, was collected before the first dose of study medication at their baseline study visit. A week-96 modified snapshot analysis excluding participants without HIV-1 RNA results after baseline also showed non-inferiority between the two treatment groups: 269 (86%) of 313 participants in the bictegravir group versus 281 (86%) of 325 in the dolutegravir group had HIV-1 RNA less than 50 copies per mL (difference -0⋅5%, 95% CI -5⋅9 to 4⋅9). The prespecified missing as excluded and missing as failure analyses were consistent with results from the full and per-protocol analyses (table 2), showing high overall efficacy and no differences between the treatment groups. The proportion of participants with HIV-1 RNA less than 20 copies per mL at week 96 by FDA snapshot algorithm was 248 (78%) of 320 for the bictegravir group and 261 (80%) of 325 for the dolutegravir group (difference -2⋅5%, 95% CI -8⋅8 to 3⋅8). Subgroup analyses showed that age, sex, race, baseline HIV-1 RNA, baseline CD4 cell count, region, and study drug adherence did not significantly influence treatment outcomes (appendix p 5). Testing for homogeneity found no significant interactions between treatment and subgroup.
Fewer participants had drug-related adverse events in the bictegravir group than in the dolutegravir group (p=0⋅02; table 3; appendix p 6). Drug-related adverse events classified as gastrointestinal disorders (MedDRA system organ class) were reported in fewer participants in the bictegravir group (29 [9%]) than in the dolutegravir group (47 [14%]) but no single adverse event (MedDRA preferred term) met the 5% threshold for between-group statistical difference (appendix p 6). No drug-related adverse events of grade 3 or higher were reported in more than 2% of participants in either group. Six participants died during the study, three (1%) participants in the bictegravir group and three (1%) in the dolutegravir group. None of the events leading to death were considered related to study drugs. There were no abnormal electrocardiogram findings associated with either treatment regimen.
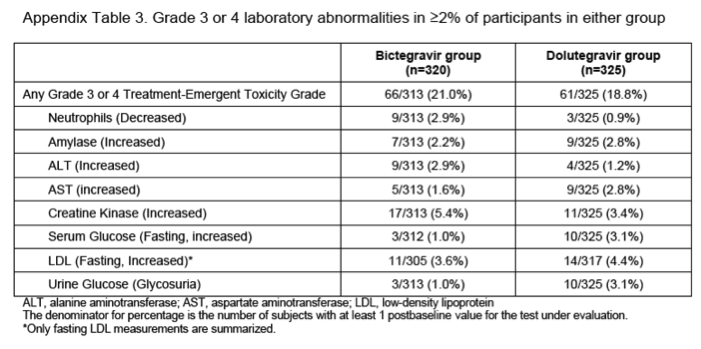
In participants with measurements available after baseline, grade 3 or 4 laboratory abnormalities were reported for 66 (21%) of 314 in the bictegravir group and 61 (19%) of 325 in the dolutegravir group (appendix p 9). Grade 3 or 4 liver-related laboratory abnormalities were uncommon and generally transient and unrelated to study treatment. Grade 3 or 4 alanine aminotransferase or aspartate aminotransferase increases were reported in ten participants in the bictegravir group and 11 participants in the dolutegravir group. The causes of these abnormalities in the bictegravir group were three hepatitis C virus infection, two hepatitis B virus infection, one alcohol abuse, one hepatitis A virus infection, two associated with creatinine kinase increase from physical exertion, and one participant had isolated grade 3 alanine aminotransferase increase without a diagnosis that resolved without interruption of study drugs. The causes of these abnormalities in the dolutegravir group were two hepatitis C virus infection, one acute hepatitis A infection, six associated with creatinine kinase increase from physical exertion, one with a history of steatohepatitis had progressive increase in both alanine aminotransferase and aspartate aminotransferase, and one participant had an isolated alanine aminotransferase increase without diagnosis that resolved without interruption of study medications. Small increases in median serum creatinine and decreases in eGFR were seen at week 96 for both groups (appendix p 10). No participants discontinued study treatment because of renal adverse events and no patients had renal tubulopathy. Changes from baseline in fasting lipid parameters were similar between groups at week 96 (appendix p 10). Initiation of lipid-modifying drugs did not differ between groups during the study: 11 (3%) of 320 in the bictegravir group and 12 (4%) of 325 in the dolutegravir group (p=1⋅00). At week 96, the median change in bodyweight in the bictegravir group was 3⋅5 kg (IQR 0⋅1–8⋅2) compared with 3⋅9 kg (0⋅8–7⋅4) in the dolutegravir group.
Both treatments were well tolerated for a median exposure of 101 weeks (IQR 98–107 for bictegravir and 98–108 for dolutegravir), with most adverse events reported as mild or moderate in severity. Adverse events leading to study drug discontinuation were uncommon, occurring in six (2%) of 320 participants in the bictegravir group and five (2%) of 325 in the dolutegravir group (table 3). No individual adverse event leading to study drug discontinuation occurred in more than one participant. Adverse event-related study drug discontinuations in the bictegravir group included one each of cardiac arrest; paranoia; chest pain; abdominal distension; sleep disorder, dyspepsia, tension headache, depressed mood, and insomnia all before week 48; and depression between weeks 48 and 96. In four of six patients with events leading to discontinuation in the bictegravir group, except cardiac arrest and paranoia (which were associated with recreational drug use), the events were considered by the investigators to be related to study drugs. Adverse events leading to study drug discontinuation in the dolutegravir group included one erythema and pruritis before week 48; and two depression; one lipoatrophy; and one supraventricular tachycardia all between weeks 48 and 96. Two of these events (one depression and one lipoatrophy) were considered related to study drugs.
14 (2%) of 645 participants had HIV-1 and hepatitis B co-infection at baseline, eight (3%) of 320 in the bictegravir group and six (2%) of 325 in the dolutegravir group. At week 96 in the missing as excluded analysis, all four (100%) participants in the bictegravir group and four (67%) of six in the dolutegravir group had hepatitis B virus DNA less than 29 IU per mL and HIV-1 RNA less than 50 copies per mL. The two participants from the dolutegravir group with hepatitis B virus DNA more than 29 IU per mL at week 96 had baseline pretreatment hepatitis B virus DNA greater than the upper limit of quantification of the assay (>170 million IU per mL) with a decrease to 55 IU per mL in one patient and 70 IU per mL in the other at week 96. Among participants without a hepatitis B virus DNA result at week 96 in the missing as excluded analysis, three (75%) of the four achieved hepatitis B virus DNA less than 29 IU per mL at their last study visit before week 96 and the remaining one had no data after baseline.
|
|
| |
| |
|
|
|