| |
Tesamorelin, liver fat, and NAFLD in the setting of HIV - Comment / Tesamorelin Prevented Progression to Liver Fibrosis in NIH Study
|
| |
| |
Download the PDF here
Download the PDF here
HIV Lancet Oct 11 2019
Jennifer Audsley, Joe Sasadeusz, *Sharon R Lewin
The Peter Doherty Institute for Infection and Immunity, The University of Melbourne and Royal Melbourne Hospital,
Melbourne, VIC 3000, Australia (JA, JS, SRL); Department of Infectious Diseases, Monash University and Alfred Hospital,
Melbourne, VIC, Australia (SRL and JS); and Victorian Infectious Disease Service, Royal Melbourne Hospital, Doherty Institute,
Melbourne, VIC, Australia (JS) sharon.lewin@unimelb.edu.au
Management of abnormal liver function tests and cirrhosis, in the absence of hepatis B or C coinfection and high alcohol intake, has become an important issue in people with HIV on antiretroviral therapy (ART). One of the most common causes for abnormal liver function in both the general population and people living with HIV is non-alcoholic fatty liver disease (NAFLD), which has two forms: non-alcoholic steatohepatitis (NASH) or hepatic steatosis (figure). Between 6% and 35% of people globally1 have NASH-related cirrhosis, and it is the fastest growing indication for liver transplantation in the USA.2
In people living with HIV on ART, the prevalence of NAFLD is higher (26-65%)3 and they can have more severe clinical and biochemical manifestations and a faster rate of liver fibrosis progression.4
Surprisingly, there are few treatment options available for this very common disease and even fewer have been evaluated in people with HIV.
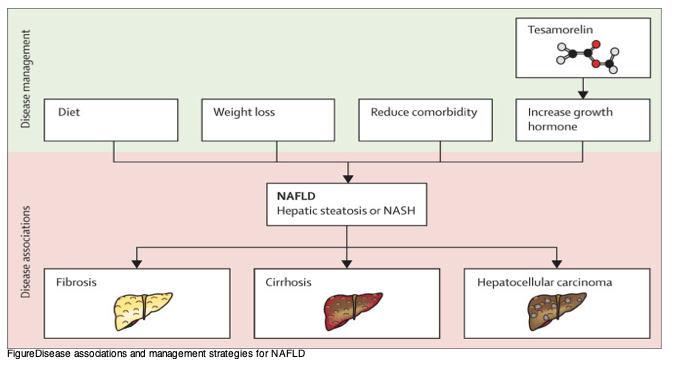
FigureDisease associations and management strategies for NAFLD
In The Lancet HIV, Takara L Stanley and colleagues5 present promising results from their randomised, double-blind, multicentre trial in which they administered daily injections of tesamorelin or placebo to people living with HIV and NAFLD.5
Tesamorelin is a synthetic peptide analogue of growth hormone-releasing hormone that restores the pulsatile release of growth hormone in people with HIV and reduces visceral abdominal fat in HIV-associated lipodystrophy.6, 7
Tesamorelin is approved by the US Food and Drug Administration to reduce visceral fat in people living with HIV and central adiposity. Tesamorelin is thought to reduce fat by stimulating lipolysis through increasing endogenous growth hormone.7
The primary endpoint of the study was change in hepatic fat fraction (HFF) after 12 months of treatment. The primary safety endpoint was blood glucose, because tesamorelin can reduce insulin sensitivity leading to hyperglycaemia. HFF was calculated following proton magnetic resonance spectroscopy (MRS) and MRI and has been shown to have a high specificity for hepatic steatosis compared with liver biopsy. An HFF of 5% or more on MRI was used to define hepatic steatosis. Additionally, a liver biopsy was done at study entry and at 12 months.
61 participants were enrolled. Compared with placebo, participants who received tesamorelin had a significant reduction in HFF with a greater proportion having a reduction of HFF to less than 5%. 38% of participants in the placebo group had liver disease progression, highlighting the importance of developing new treatment strategies for NAFLD. Tesamorelin prevented the progression of liver fibrosis during the treatment period but did not improve existing fibrosis. There was no significant effect of tesamorelin on liver enzymes or blood lipids. Finally, although tesamorelin was well tolerated with no overall change in fasting glucose or glycated haemoglobin, there were two study discontinuations in the tesamorelin group due to hyperglycaemia.
Meta-analyses8 of NAFLD in HIV have identified associations with traditional risk factors rather than HIV-related risk factors or antiretroviral drugs. The current approach to managing NAFLD and NASH is primarily related to lifestyle changes, including treatment of comorbidities such as diabetes, hypertension, dyslipidaemias, and reducing the cardiovascular risk profile. In the general population, a weight loss of 5-10% of body mass is recommended, but it is unclear if this amount of weight loss will also lead to improved outcomes for people living with HIV.
Because there are very few therapeutic drugs available for NASH or NAFLD, Stanley and colleagues' study 5 is an important therapeutic advance. However, several issues require further consideration. First, it is unclear what will happen after stopping tesamorelin, specifically whether declines in the HFF will be maintained. Second, if tesamorelin is required for a longer duration, there are no long-term data on safety. Third, tesamorelin requires refrigeration and access to a supply of clean needles and syringes, which could reduce its use in some low-income and middle-income countries, where NAFLD prevalence is substantial and increasing, such as in India. 9
Finally, tesamorelin had no effect on participants with established fibrosis at entry, which means that the greatest clinical benefit of the drug will be achieved through early detection of NAFLD. Increased investment will be needed to increase the awareness of the condition to allow for early diagnosis and a focus on prevention. Tesamorelin provides a new therapeutic approach that is important to managing people living with HIV and NAFLD and warrants further investigation.
We declare no competing interests.
--------------------------
Our data show that tesamorelin reduces liver fat content in people with HIV and NAFLD and further suggest it prevents a high rate of progression of fibrosis, and improves inflammatory indices, such as CRP. In HIV-positive individuals recruited for NAFLD, we saw a broad range of baseline NAS scores, and patients given tesamorelin with higher NAS scores at baseline had greater reductions in NAS score with tesamorelin. Additionally, in individuals with elevated ALT at baseline, ALT decreased with tesamorelin. Importantly, tesamorelin did not worsen insulin sensitivity. Collectively, these data suggest a significant benefit of tesamorelin among people with HIV and NAFLD. Tesamorelin is FDA approved to reduce visceral fat in people with HIV with central adiposity. Our results now suggest that it might be beneficial among the larger group of people with HIV and NAFLD.
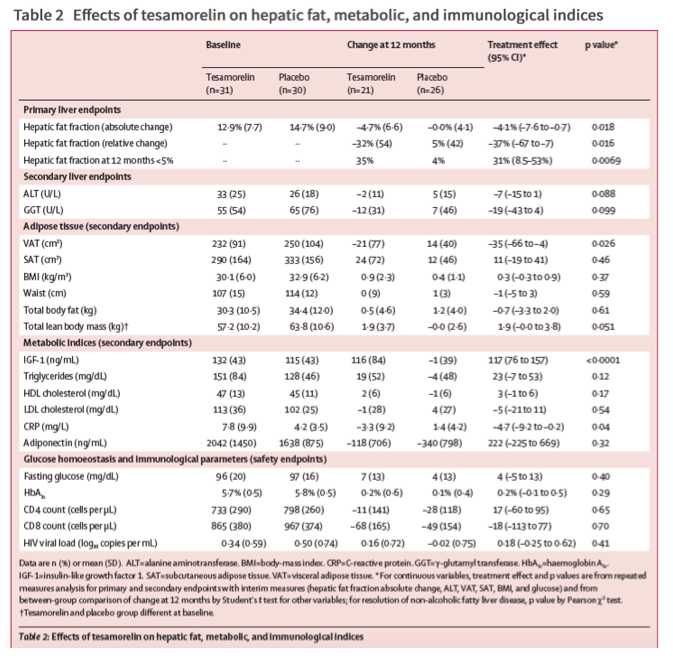
The mean adherence to daily injections by count of returned empty vials was similar between treatment groups: 87% (SD 16) for placebo and 80% (15) for tesamorelin (p=0⋅11). IGF-1 concentrations increased in the tesamorelin group (effect size 117 ng/mL, 95% CI 76-157, p<0⋅0001; table 2). No participants had IGF-1 Z scores over the prespecified dose-reduction threshold, but one participant received an institutional review board-approved dose reduction to 1 mg for symptoms potentially related to growth hormone. This individual self-discontinued from the study soon after the dose reduction.
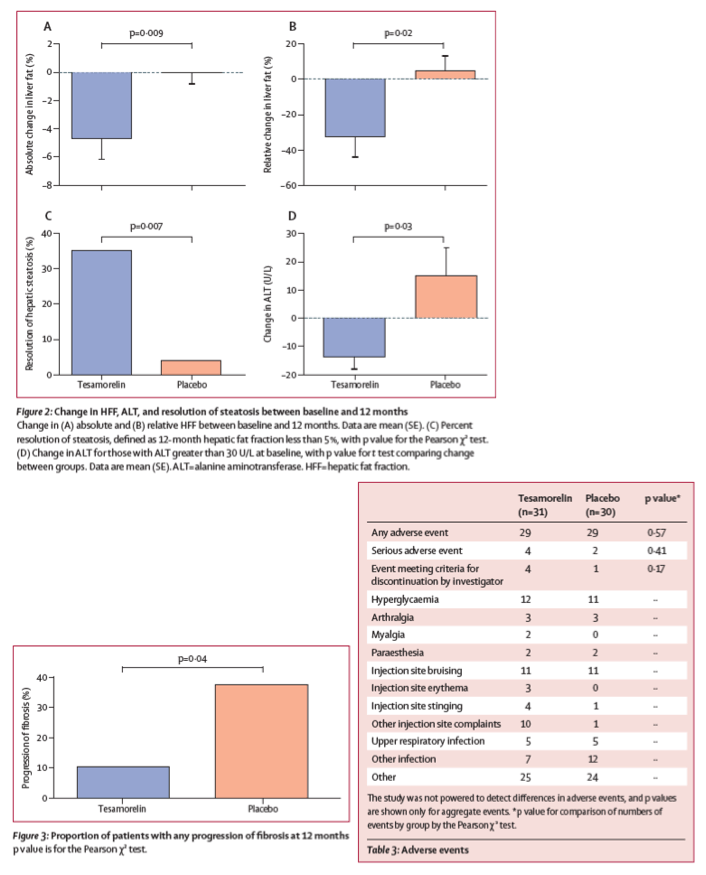
Tesamorelin prevented the progression of fibrosis during the treatment period, with two (10⋅5%) individuals showing progression in the tesamorelin group versus nine (37⋅5%) individuals in the placebo group (p=0⋅044; figure 3). However, tesamorelin did not improve existing fibrosis: among those with fibrosis stage 1 or higher at baseline, fibrosis improved in two individuals in the tesamorelin group and three individuals in the placebo group (p=0⋅71). Changes in fibrosis during the study were positively associated with changes in NAS score (p=0⋅0003 for ANOVA). When compared with placebo, tesamorelin did not significantly change NAS score (effect size -0⋅3, 95% CI -1⋅0 to 0⋅5), lobular inflammation score (-0⋅3, -0⋅7 to 0⋅2), or hepatocellular ballooning (-0⋅1, -0⋅4 to 0⋅2). In exploratory analyses, the higher the baseline NAS score, more change was seen among the tesamorelin-treated individuals (r=-0⋅48, p=0⋅040), whereas a similar relationship was not observed in the placebo group (r=-0⋅14, p=0⋅52).
Compared with placebo, tesamorelin did not significantly reduce ALT or γ-glutamyl transferase over the treatment period, although both effect sizes suggest a modest reduction (table 2). Restricting the cohort to those with elevated ALT (>30 U/L) at baseline, tesamorelin did significantly decrease ALT after 12 months (effect size -29 U/L, 95% CI -55 to -3; figure 2) compared with placebo. Tesamorelin reduced CRP (effect size -4⋅7 mg/L, 95% CI -9⋅2 to -0⋅2; table 2) compared with placebo but did not have any effect on adiponectin.
In our study we did not see any effect of tesamorelin on LDL cholesterol, HDL cholesterol, or triglycerides (table 2). Tesamorelin did not significantly affect fasting glucose or HbA1c during the treatment period (table 2).
Tesamorelin significantly reduced VAT area compared with placebo, with no effect on SAT area (table 2). Tesamorelin modestly increased lean body mass by DXA, with no significant effect on total body fat mass (table 2). BMI and waist circumference did not change.
-----------------------------
NIAID - Drug Reverses Signs of Liver Disease in People Living with HIV
Tesamorelin Prevented Progression to Liver Fibrosis in NIH Study
October 11, 2019
https://www.niaid.nih.gov/news-events/drug-reverses-signs-liver-disease-people-living-hiv
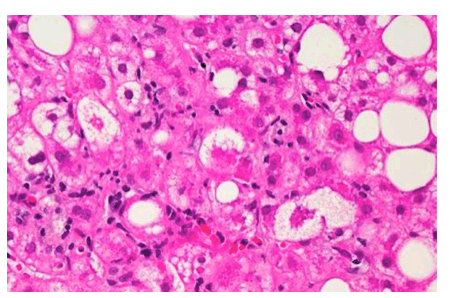
A microscopic image of liver tissue affected by non-alcoholic fatty liver disease (NAFLD). The large and small white spots are excess fat droplets filling liver cells (hepatocytes).
Credit: Dr. David Kleiner, NCI
Researchers at the National Institutes of Health and their colleagues at Massachusetts General Hospital (MGH) in Boston report that the injectable hormone tesamorelin reduces liver fat and prevents liver fibrosis (scarring) in people living with HIV. The study was conducted by the National Institute of Allergy and Infectious Diseases (NIAID) and the National Cancer Institute, both parts of NIH. The findings were published online today in The Lancet HIV.
"Many people living with HIV have overcome significant obstacles to live longer, healthier lives, though many still experience liver disease," said NIAID Director Anthony S. Fauci, M.D. "It is encouraging that tesamorelin, a drug already approved to treat other complications of HIV, may be effective in addressing non-alcoholic fatty liver disease."
Non-alcoholic fatty liver disease, or NAFLD, frequently occurs alongside HIV, affecting as many as 25% of people living with HIV in the developed world. However, no effective treatments currently exist to treat the condition, which is a risk factor for progressive liver disease and liver cancer. Investigators led by Colleen M. Hadigan, M.D., senior research physician in NIAID's Laboratory of Immunoregulation, and Steven K. Grinspoon, M.D., Chief of the Metabolism Unit at MGH, tested whether tesamorelin could decrease liver fat in men and women living with both HIV and NAFLD. Among the participants enrolled, 43% had at least mild fibrosis, and 33% met the diagnostic criteria for a more severe subset of NAFLD called nonalcoholic steatohepatitis (NASH). Thirty-one participants were randomized to receive daily 2-mg injections of tesamorelin, and 30 were randomized to receive identical-looking injections containing a placebo. Researchers provided nutritional counseling to all participants, as well as training in self-administering the daily injections. Researchers then compared measures of liver health in both groups at baseline and 12 months.
After one year, participants receiving tesamorelin had better liver health than those receiving placebo, as defined by reduction in hepatic fat fraction (HFF)-the ratio of fat to other tissue in the liver. The healthy range for HFF is less than 5%. Thirty-five percent of study participants receiving tesamorelin achieved a normal HFF, while only 4% of those on placebo reached that range with nutritional advice alone. Overall, tesamorelin was well-tolerated and reduced participants' HFF by an absolute difference of 4.1% (corresponding to a 37% relative reduction from the beginning of the study). While nine participants receiving placebo experienced onset or worsening of fibrosis, only two participants in the tesamorelin group experienced the same. Additionally, levels of several blood markers associated with inflammation and liver damage-including the enzyme alanine aminotransferase (ALT)-decreased more among those taking tesamorelin compared to those on a placebo, particularly among those with increased levels at the beginning of the study.
Given these positive results, investigators suggest expanding the indication for tesamorelin to include people living with HIV who have been diagnosed with NAFLD. They also recommend additional research to determine if tesamorelin could contribute to long-term protection against serious liver disease in people without HIV.
"Our hope is that this intervention may help people living with HIV, as well as benefit HIV-negative people with liver abnormalities," said Dr. Hadigan. "Further research may inform us of the potential long-term benefits of this approach and develop formulations that can benefit everyone with liver disease, regardless of HIV status."
Egrifta (tesamorelin) was approved in 2010 by the U.S. Food and Drug Administration to reduce excess abdominal fat in HIV patients with lipodystrophy-a complication characterized by an abnormal distribution of body fat initially associated with older classes of HIV medications. The most commonly reported side effects in previous clinical trials evaluating Egrifta included joint pain (arthralgia), skin redness and rash at the injection site (erythema and pruritis), stomach pain, swelling, and muscle pain (myalgia). Worsening blood sugar control occurred more often in trial participants treated with Egrifta than with placebo.
"Because tesamorelin proved effective in treating abnormal fat build-up in the abdomens of people in the context of HIV and related medication use, we hypothesized that the drug might also reduce fat that accrues in the liver and causes damage in a similar population," said Dr. Grinspoon.
While liver disease is often associated with heavy alcohol use, NAFLD occurs when excess fat builds up in the liver without alcohol as a contributing factor. This condition may progress to liver damage, cirrhosis or cancer that could be life-threatening and necessitate liver transplantation.
Previous studies have found that vitamin E supplements, weight loss and other lifestyle changes can improve outcomes among HIV-negative people with NASH. However, treatment options for NASH and NAFLD are often not tested in people with HIV and none are available for this group. Obesity and type 2 diabetes raise the risk of developing NAFLD regardless of HIV status, and people with HIV are at increased risk of NAFLD because some HIV medications and HIV itself are associated with gaining abdominal fat and may contribute to liver fat build-up.
This research was supported through NIAID grant U01 AI115711. For more information about this trial, please visit ClinicalTrials.gov under study identifier NCT02196831.
Reference: T Stanley et al. Effects of tesamorelin on nonalcoholic fatty liver disease in HIV: a randomized, double-blind, multicenter trial. The Lancet HIV DOI: 10.1016/PII (2019).
--------------------------------------
Effects of tesamorelin on non-alcoholic fatty liver disease in HIV: a randomised, double-blind, multicentre trial
Lancet HIV
Takara L Stanley*, Lindsay T Fourman*, Meghan N Feldpausch, Julia Purdy, Isabel Zheng, Chelsea S Pan, Julia Aepfelbacher, Colleen Buckless,
Andrew Tsao, Anela Kellogg, Karen Branch, Hang Lee, Chia-Ying Liu, Kathleen E Corey, Raymond T Chung, Martin Torriani, David E Kleiner,
Colleen M Hadigan†, Steven K Grinspoon†
Summary
Background
Non-alcoholic fatty liver disease (NAFLD) is a substantial cause of comorbidity in people with HIV and there are no proven pharmacological treatments for the disease in this population. We assessed the effects of tesamorelin on liver fat and histology in people with HIV and NAFLD.
Methods
This randomised, double-blind, multicentre study with identical placebo as a comparator was done in a hospital and a medical research centre in the USA. People with HIV infection and a hepatic fat fraction (HFF) of 5% or more by proton magnetic resonance spectroscopy were eligible. Participants were randomly assigned (1:1) to receive either tesamorelin 2 mg once daily or placebo once daily for 12 months, followed by a 6-month open-label phase during which all participants received tesamorelin 2 mg daily. The randomisation list was prepared by the study statistician using a permuted block algorithm within each stratum with randomly varying block sizes. The primary endpoint was change in HFF between baseline and 12 months. The primary safety endpoint was glucose. Analysis was by intention to treat using all available data. This trial is registered with ClinicalTrials.gov, number NCT02196831.
Findings
61 patients were enrolled between Aug 20, 2015, and Jan 16, 2019, of whom 30 received tesamorelin and 30 received placebo. Patients receiving tesamorelin had a greater reduction of HFF than did patients receiving placebo, with an absolute effect size of -4⋅1% (95% CI -7⋅6 to -0⋅7, p=0⋅018), corresponding to a -37% (95% CI -67 to -7, p=0⋅016) relative reduction from baseline. After 12 months, 35% of individuals receiving tesamorelin and 4% receiving placebo had a HFF of less than 5% (p=0⋅0069). Changes in fasting glucose and glycated haemoglobin were not different between groups at 12 months. Individuals in the tesamorelin group experienced more localised injection site complaints than those in the placebo group, though none were judged to be serious.
Interpretation
Tesamorelin might be beneficial in people with HIV and NAFLD. Further studies are needed to determine the long-term effects of tesamorelin on liver histology.
Funding
National Institutes of Health and National Institute of Allergy and Infectious Diseases.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is defined by excess storage of triglyceride in hepatocytes (steatosis) and is often accompanied by inflammation, cellular damage, and fibrosis. The development of ballooning hepatocellular injury shows progression to non-alcoholic steatohepatitis (NASH). NASH can progress to cirrhosis and is an increasingly important cause of end-stage liver disease in the general population. NAFLD can be more prevalent in people with HIV than in the general population, and studies suggest that progression of fibrosis is more likely in people with HIV.1, 2, 3
Unlike many HIV-associated comorbidities that worsen with increased severity of HIV disease, NAFLD can occur more commonly in those with well treated HIV and higher CD4 T-cell counts and weight gain, often in association with central adiposity.4, 5, 6
In people with HIV, weight gain, abdominal fat accumulation, and increases in visceral fat are common and seen even with newer antiretrovirals.7
There are no proven therapies for NAFLD in people with HIV, nor is it known how strategies to reduce liver fat would affect progression of histological changes over time, and thus alter the natural history among people with HIV.
Growth hormone secretory dynamics are perturbed with reduced pulsatile growth hormone among people with HIV. The degree of perturbation closely parallels abdominal fat accumulation and weight gain.8, 9, 10
Tesamorelin is a growth hormone-releasing hormone (GHRH) analogue that restores endogenous pulsatile growth hormone secretion and reduces visceral fat in individuals with HIV infection.11, 12, 13
Tesamorelin is thought to stimulate lipolysis via increased endogenous growth hormone while maintaining feedback inhibition and limiting toxicity compared with growth hormone per se.13
We previously showed that tesamorelin reduces liver fat content in a preliminary study of people with HIV chosen for abdominal obesity.14
This current study was designed to substantially extend these data and address an important question for people with HIV. Our study assessed for the first time a therapeutic strategy specifically for people with HIV and NAFLD; and simultaneously assessed changes in liver histopathology, inflammatory, and metabolic indices, including specific indices of insulin sensitivity by euglycaemic hyperinsulinaemic clamp.
Compared with placebo, tesamorelin did not significantly reduce ALT or γ-glutamyl transferase over the treatment period, although both effect sizes suggest a modest reduction (table 2). Restricting the cohort to those with elevated ALT (>30 U/L) at baseline, tesamorelin did significantly decrease ALT after 12 months (effect size -29 U/L, 95% CI -55 to -3; figure 2) compared with placebo. Tesamorelin reduced CRP (effect size -4⋅7 mg/L, 95% CI -9⋅2 to -0⋅2; table 2) compared with placebo but did not have any effect on adiponectin.
In our study we did not see any effect of tesamorelin on LDL cholesterol, HDL cholesterol, or triglycerides (table 2). Tesamorelin did not significantly affect fasting glucose or HbA1c during the treatment period (table 2). Fasting glucose at each study visit for each group is shown in the appendix (p 3. In the subset of patients at Massachusetts General Hospital who underwent euglycaemic hyperinsulinaemic clamp, tesamorelin did not affect the insulin-stimulated glucose uptake (M) during the low-dose clamp (treatment effect for low-dose M 0⋅0 mg/kg per min, 95% CI -1⋅1 to 1⋅1, p>0⋅99) nor the insulin stimulated glucose uptake during the high-dose clamp (treatment effect for high-dose M -0⋅9 mg/kg per min, 95% CI -2⋅4 to 0⋅7, p=0⋅27).
Tesamorelin significantly reduced VAT area compared with placebo, with no effect on SAT area (table 2). Tesamorelin modestly increased lean body mass by DXA, with no significant effect on total body fat mass (table 2). BMI and waist circumference did not change.
There were no significant changes in baseline and 12-month estimates of daily caloric and macronutrient intake by 4-day food record, self-reported alcoholic drinks per week, and hours of activity per week as assessed by the Modifiable Activity Questionnaire (appendix p ).
Sensitivity analyses for the primary endpoint were done using multiple imputation for missing data. These data confirmed the primary results, with an estimated effect size of -3⋅8% (95% coverage interval -5⋅5 to -2⋅2) reduction in HFF.
A small number of serious adverse events were seen, which did not differ by treatment group, and were judged as unrelated to study drug (table 3). In the placebo group, two individuals were hospitalised with serious adverse events: one following a suicide attempt, and one following a haematoma after a liver biopsy. In the tesamorelin group, four individuals were hospitalised with serious adverse events: one with transient hemiplegia for which a cause was not found, one due to suicidal ideation, one for urosepsis, and one for pneumonia and separately for influenza.
One individual in the placebo group and four in the tesamorelin group had events that met a-priori protocol criteria for investigator discontinuation (p=0⋅17), including two discontinuations for hyperglycaemia (2-week and 6-month visits) in the tesamorelin group. One had known diabetes at baseline. Other reasons for discontinuation are outlined in figure 1.
Discussion
Our data show that tesamorelin reduces liver fat content in people with HIV and NAFLD and further suggest it prevents a high rate of progression of fibrosis, and improves inflammatory indices, such as CRP. In HIV-positive individuals recruited for NAFLD, we saw a broad range of baseline NAS scores, and patients given tesamorelin with higher NAS scores at baseline had greater reductions in NAS score with tesamorelin. Additionally, in individuals with elevated ALT at baseline, ALT decreased with tesamorelin. Importantly, tesamorelin did not worsen insulin sensitivity. Collectively, these data suggest a significant benefit of tesamorelin among people with HIV and NAFLD.
In the larger context of comorbidities not related to AIDS, chronic liver disease causes substantial morbidity and mortality in people with HIV.18
Although estimates of prevalence vary from 13% to 60%, NAFLD is likely to be present in 25% or more of people with HIV, with an increased prevalence in those with visceral or generalised obesity.6, 19, 20
NAFLD is expected to soon become the leading cause of liver-related morbidity and mortality in people with HIV.21
In the general population, lifestyle modification leads to reduction in liver fat content and amelioration of steatohepatitis and is currently the cornerstone of therapy for NAFLD.22
However, weight loss of up to 5-10% might be needed in this regard, and data from the general population might not be generalisable to the HIV population, in whom ectopic adipose tissue, dysfunctional subcutaneous adipose tissue, and excessive immune activation are all seen.22, 23
At present, there are no widely accepted pharmacological strategies to treat NAFLD and NASH. Vitamin E reduces ALT and improves histological features of inflammation, and some organisations recommend it for non-diabetic adults with NASH in the general population, but there is concern from meta-analyses that long-term use could modestly increase all-cause mortality.22, 24
Pioglitazone, a peroxisome proliferator-activated receptor γ agonist, also improves features of steatohepatitis, but use is typically accompanied by weight gain.24
Obeticholic acid is a farnesoid X receptor agonist that improved steatohepatitis in earlier trials but also increased LDL cholesterol and decreased HDL cholesterol.25
A phase 3 study's results are consistent with earlier trials, both in terms of improvement in steatohepatitis and increase in LDL cholesterol.26
Other drugs with varying mechanisms of action are being investigated, but none have thus far been approved. Moreover, with few exceptions, these studies have been done in the general population, and data for the HIV population are insufficient.
Considering our results in the context of existing and emerging treatment strategies, tesamorelin is the only drug thus far to show efficacy in reducing liver fat and preventing fibrosis progression specifically in HIV. To our knowledge, the only other pharmacological strategy that has been specifically tested for NAFLD in people with HIV is the bile acid conjugate aramchol, which did not reduce liver fat in 50 people with HIV and lipodystrophy that were given the drug for 12 weeks.27
Tesamorelin is FDA approved to reduce visceral fat in people with HIV with central adiposity. Our results now suggest that it might be beneficial among the larger group of people with HIV and NAFLD.
The mechanisms whereby liver steatosis in NAFLD progresses to fibrosis and steatohepatitis are not known, nor is the natural history of these changes well defined in longitudinal studies among people with HIV. The data from our study show for the first time that a strategy aimed mechanistically at reducing liver fat has a significant effect to prevent changes in liver histopathology in people with HIV. Tesamorelin can reduce liver fat via multiple mechanisms. One of the most important physiological effects of growth hormone is to increase the activity of hormone-sensitive lipase, an important lipolytic enzyme present in adipose tissue, liver, muscle, and other tissues. With the data available, it is unclear if increased lipolysis in the liver, adipose tissue, or both are responsible for net reductions in liver fat, and the tissue-specific mechanisms of tesamorelin require further study.
Fibrosis stage is the strongest predictor of mortality in patients with NAFLD, and FDA guidance on treatments for NAFLD stresses the importance not only of liver fat reduction, but also of prevention of fibrosis and reduction of inflammation.28
We did liver biopsies before and after treatment and they showed that tesamorelin prevented progression of fibrosis. Moreover, reductions in liver fat were associated with reductions in fibrosis in a population with strictly defined NAFLD. Such an effect might prevent the development of cirrhosis in people with HIV. Future studies are needed to further explore clinical outcomes in the context of liver fat reduction and progression of fibrosis in this population. Our data suggest a large percentage (38%) of HIV patients with NAFLD in the placebo group show progression of fibrosis over 1 year. This is an important finding and highlights the crucial need for a therapy to prevent fibrosis progression in this population.
In this study, two individuals in the tesamorelin group, one with baseline diabetes, required discontinuation for worsening hyperglycaemia. However, among the entire cohort, tesamorelin was neutral to glucose homoeostasis over the 12-month study period, and clinically relevant hyperglycaemia events were well balanced between the placebo and tesamorelin groups. These results are consistent with our previous studies, which show a modest worsening of insulin resistance in the first few weeks to months of tesamorelin treatment, followed by a return to baseline over longer-term therapy.14, 29
In previous studies aggregating phase 3 clinical trial data, the degree of VAT reduction was associated with lowering of HbA1c,29 suggesting that ongoing reduction in ectopic fat, as well as increasing lean body mass, can ultimately counterbalance initial increases in glucose. Nonetheless, caution should be used in patients with NAFLD and significant baseline dysglycaemia, and a minority of patients might not be able to continue tesamorelin due to hyperglycaemia. Clinically, tesamorelin has been safely used in the population with HIV for almost a decade, but patients with NAFLD can have more severe glucose derangements, and assessment of glucose early after intervention in such patients is prudent. Importantly, we did not test whether simultaneous antidiabetic strategies to lower glucose and insulin might be useful to allow the small subset experiencing dysglycaemia to continue with tesamorelin and maintain normoglycaemia. Future studies should address this issue, because of the potential value of reducing fibrosis progression in these patients.
Our study was randomised, placebo-controlled, and included gold standard imaging before and after biopsy sampling, as well the performance of euglycaemic hyperinsulinaemic clamp. We recruited for NAFLD as prespecified in the study protocol, and the study was powered a priori to detect changes in liver fat. By contrast, the study was not designed as a therapeutic trial for NASH, not all patients had steatohepatitis, and there was a range of baseline NAS scores.
Therefore, we could not definitively assess effects on endpoints recommended by the US FDA as appropriate for late-stage studies of NASH therapeutics, namely improvements in histological evidence of fibrosis, or inflammation, or both. Further studies specifically recruiting for NASH are now indicated. Our data demonstrate an effect of tesamorelin to prevent progression of fibrosis among people with HIV and NAFLD, with strong relationships between reductions in liver fat and fibrosis. Importantly, this suggests that the strategy of tesamorelin, aimed mechanistically at reducing liver fat, might be useful to prevent liver fibrosis in people with HIV. Studies of individuals achieving visceral fat reduction with tesamorelin show that visceral fat reaccumulates after discontinuation of treatment, 30 and studies investigating the use of tesamorelin in NAFLD and NASH will need to investigate whether effects are durable beyond the treatment period.
In conclusion, our data show that tesamorelin robustly decreases liver fat, while preventing fibrosis progression, in association with improvement in indices of liver inflammation among people with HIV and NAFLD. The treatment is generally well tolerated, and future studies are needed to further define the clinical role of tesamorelin with respect to NAFLD in people with HIV, and potentially other populations.
|
|
| |
| |
|
|
|