 |
 |
 |
| |
Oral Cabotegravir and Rilpivirine Durable for 5.5 Years in LATTE Trial
|
| |
| |
IDWeek, October 2-6, 2019, Washington, DC
Mark Mascolini
Oral cabotegravir (CAB) and rilpivirine (RPV) taken for 288 weeks proved a durable and largely well-tolerated 2-drug maintenance regimen after 24 weeks of CAB plus 2 nucleosides in the venerable LATTE trial [1]. Researchers reported only 8 protocol-defined virologic failures in 160 people (5%) who started CAB+RPV.
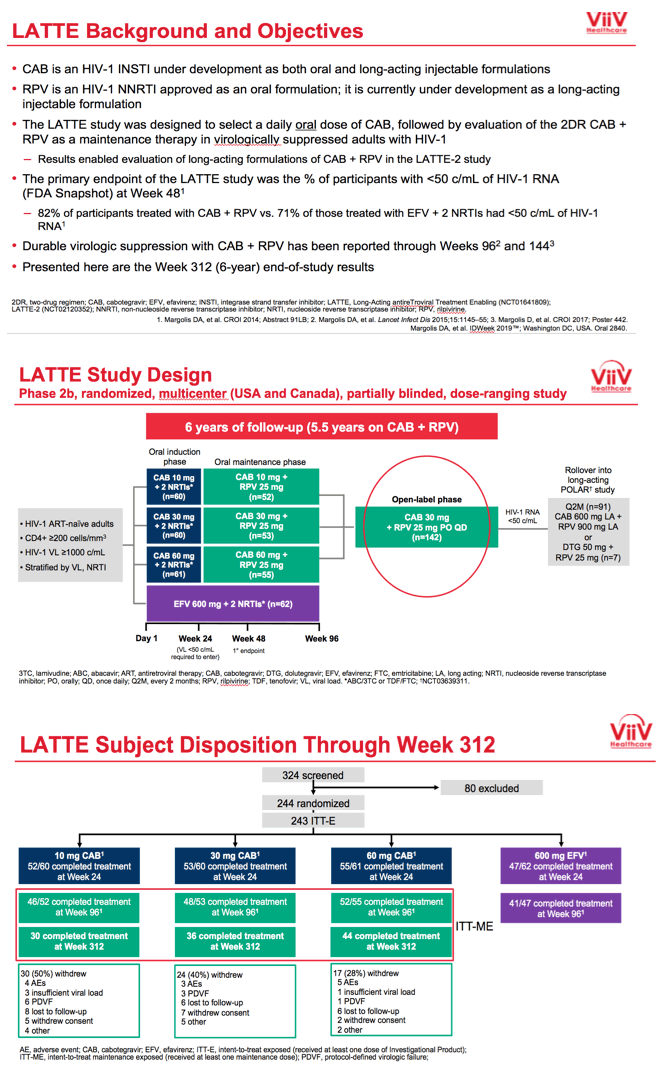
ViiV Healthcare researchers planned LATTE to pick an oral dose of the integrase inhibitor CAB and to test an oral CAB+RPV combination over several years of treatment. Development of CAB continues as both an oral drug and a long-acting injectable given with injectable RPV in LATTE-2 and other trials.
LATTE enrolled antiretroviral-naive adults and randomized them in a 1:1:1:1 ratio to oral CAB at 10, 30, or 60 mg once daily or to 600 mg of efavirenz once daily, each with tenofovir/emtricitabine or abacavir/lamivudine. CAB recipients who had a viral load below 50 copies immediately before study week 24 replaced their nucleosides with 25 mg or oral RPV and continued 10, 30, or 60 mg of CAB through 96 weeks. People randomized to efavirenz continued their 3-drug regimen. At week 96 efavirenz-treated people left the study, while the CAB+RPV group got the option to continue open-label once-daily CAB at 30 mg plus RPV at 25 mg through study week 312. Thus participants could take oral CAB+RPV for 288 weeks.
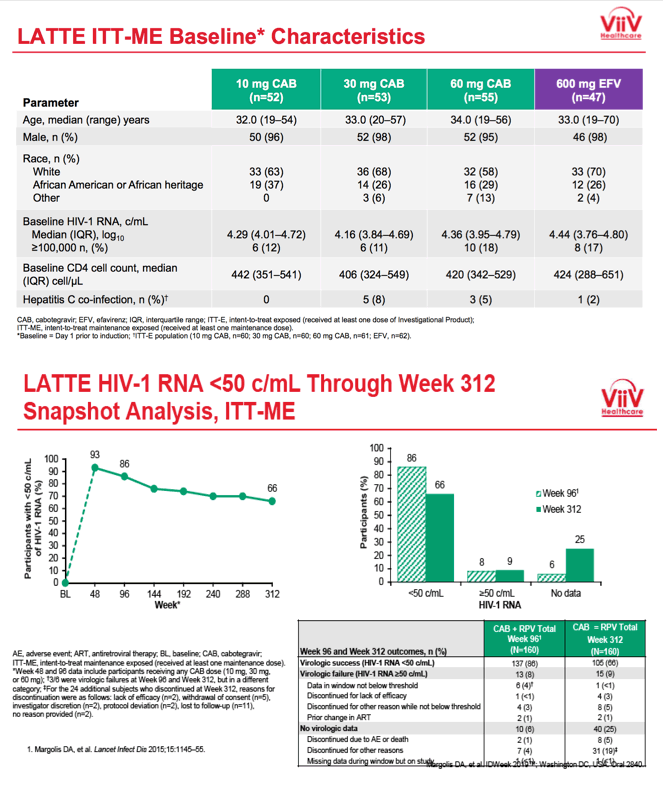
Seven years ago, when LATTE began, median ages across the four treatment groups ranged from 32 to 34. Almost all participants--95% or more in each group--were men, about two thirds were white and about 30% of African heritage. Across the four treatment groups, 11% to 18% had a pretreatment viral load above 100,000 copies.
In LATTE's primary endpoint, FDA snapshot analysis at week 48 determined that 82% randomized to CAB+RPV versus 71% randomized to an efavirenz combination had a viral load below 50 copies. In the open-label phase starting at 96 weeks, 142 people continued CAB+RPV.
At week 312 an intention-to-treat analysis among 160 participants who took at least one maintenance dose of CAB+RPV determined that 66% had a viral load below 50 copies, 9% had a load above 50 copies, and 25% had no virologic data. Among the 15 people with virologic failure at week 312, 4 (2.5% of 160) discontinued for lack of efficacy and 8 (5%) discontinued for other reasons while not below 50 copies.
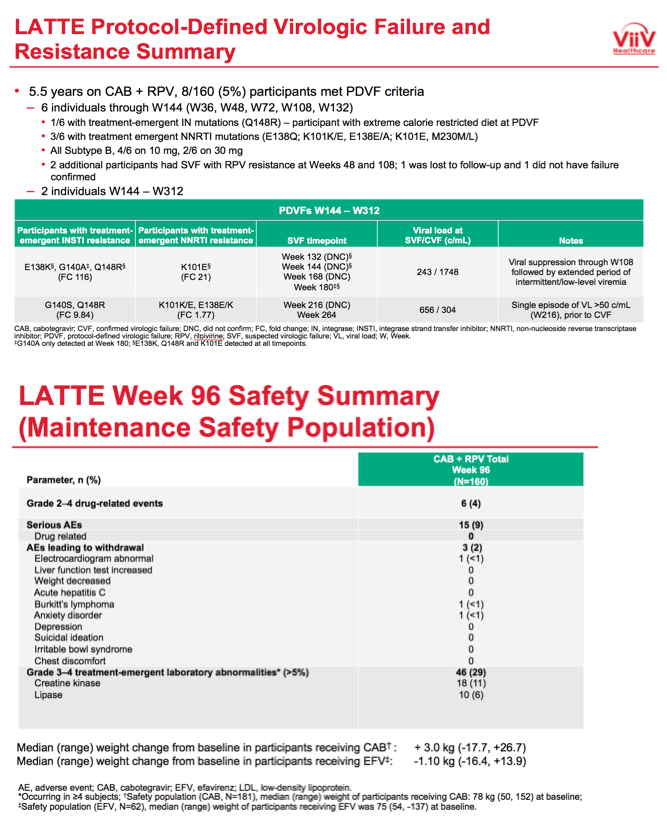
After taking CAB+RPV for 5.5 years, 8 of 160 people (5%) had protocol-defined virologic failure. Six of these 8 met virologic failure criteria through week 144, 3 of whom had treatment-emergent nonnucleoside mutations and 1 of whom had treatment-emergent integrase inhibitor mutations. The 2 people who had protocol-defined virologic failure between weeks 144 and 312 both had treatment-emergent integrase inhibitor and nonnucleoside mutations.
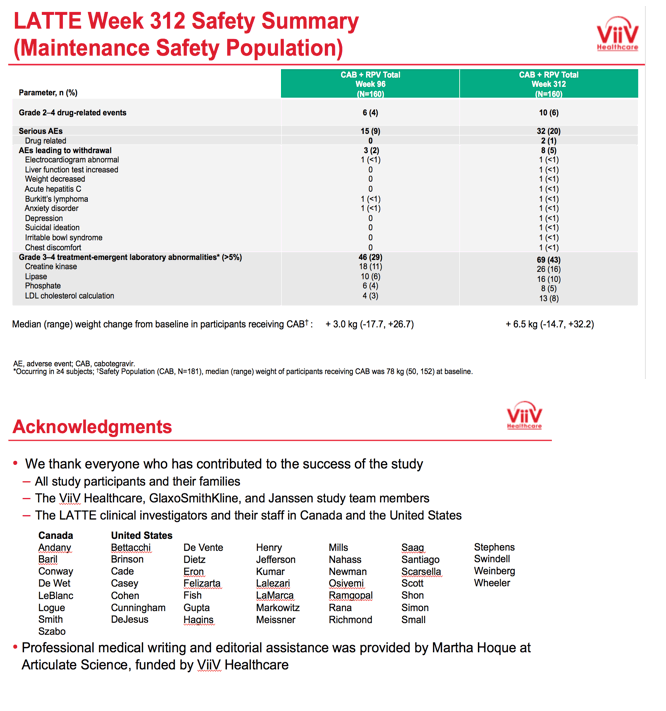
Among 160 people who took maintenance CAB+RPV, after 312 weeks 10 (6%) had grade 2 to 4 drug-related events, 8 (5%) had adverse events leading to withdrawal, 32 (20%) had serious adverse events, 2 (1%) had serious drug-related adverse events, and 69 (43%) had grade 3 or 4 treatment-emergent lab abnormalities (including 26 creatine kinase, 16 lipase, 13 LDL cholesterol, and 8 phosphate abnormalities). People taking CAB through week 312 gained a median of 6.5 kg (range -14.7 to +32.2 kg) since entering the trial. Median weight gain at week 96 had been 3.0 kg.
Reference
1. Margolis DA, Sutton KC, De Vente J, et al. Long term efficacy, safety and durability of CAB and RPV as two drug oral maintenance therapy--LATTE week 312 results. IDWeek, October 2-6, 2019, Washington, DC. Abstract 2840.
|
| |
|
 |
 |
|
|