 |
 |
 |
| |
The risk of HCV recurrence in HCV infected patients treated with DAA after achieving a sustained virological response: a comprehensive analysis
|
| |
| |
AASLD 2020 virtual Nov 11-16
Reported by Jules Levin
Peng Huang1,2, Yan Wang2, Jacinta A Holmes3, Tuo Shao1, Zhimeng Cheng1, Jiuliang Yan1, Yanzhang Tian1, Rongbin Yu2, Andre Jeyarajan1, Chuanwu Zhu4, Sheng Yang2, Wenyu Lin1and Raymond T. Chung1
1. Massachusetts General Hospital and Harvard Medical School
2. School of Public Health, Nanjing Medical University, China
3. St Vincent's Hospital, University of Melbourne, Australia
4. The Fifth People's Hospital of Suzhou, China
abstract
THE RISK OF HCV RECURRENCE IN HCV INFECTED PATIENTS TREATED WITH DAA AFTER ACHIEVING A SUSTAINED VIROLOGICAL RESPONSE: A COMPREHENSIVE ANALYSIS
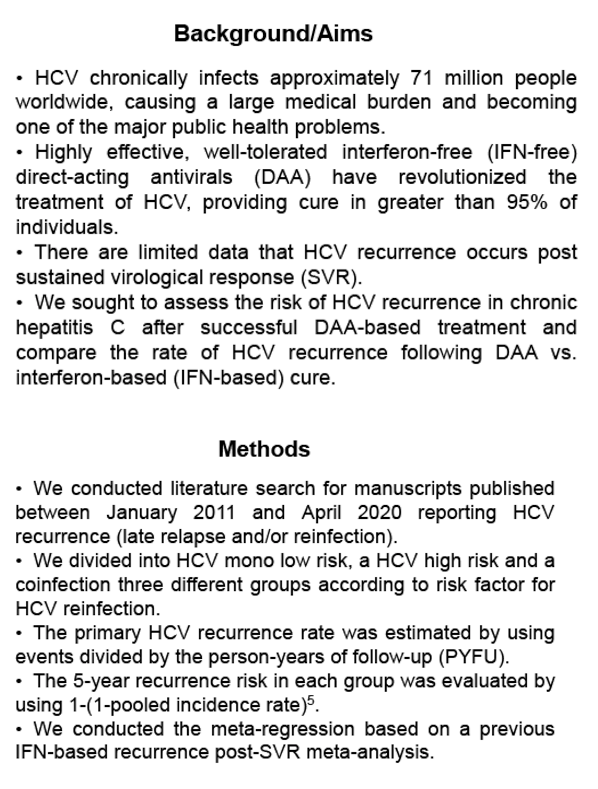
Background: Direct-acting antivirals (DAA) are highly effective, well-tolerated, and short duration regimens for the treatment of patients with chronic hepatitis C virus (HCV) infection . Currently, there are limited data that recurrence occurs after sustained virological response (SVR) . Therefore, we conducted a systematic analysis in order to assess the risk of HCV recurrence in chronic hepatitis C after successful DAA-based treatment.
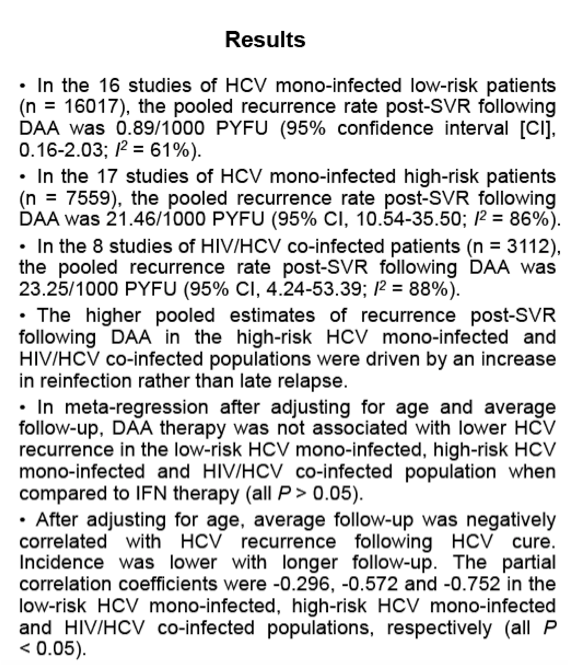
Methods: A search was conducted for manuscripts published between January 2011 and April 2020 reporting HCV recurrence (late relapse and/or reinfection) rates. Identified publications were then divided into three different groups according to risk factor for HCV reinfection: low risk HCV mono-infection, high risk HCV mono-infection and HIV/HCV coinfection . The primary HCV recurrence rate was estimated by using events divided by the person-years of follow-up (PYFU) . The 5-year recurrence risk in each group was evaluated by using 1-(1-pooled incidence rate)5. HCV recurrence was defined as confirmed detectable HCV RNA following documented SVR with DAA therapy.
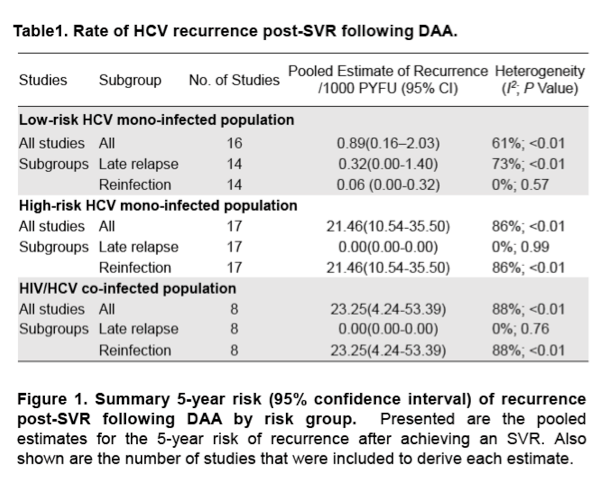
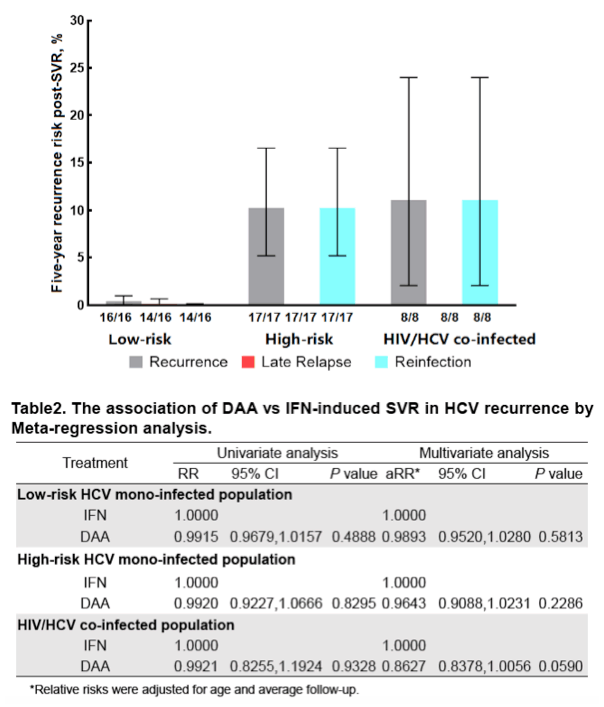
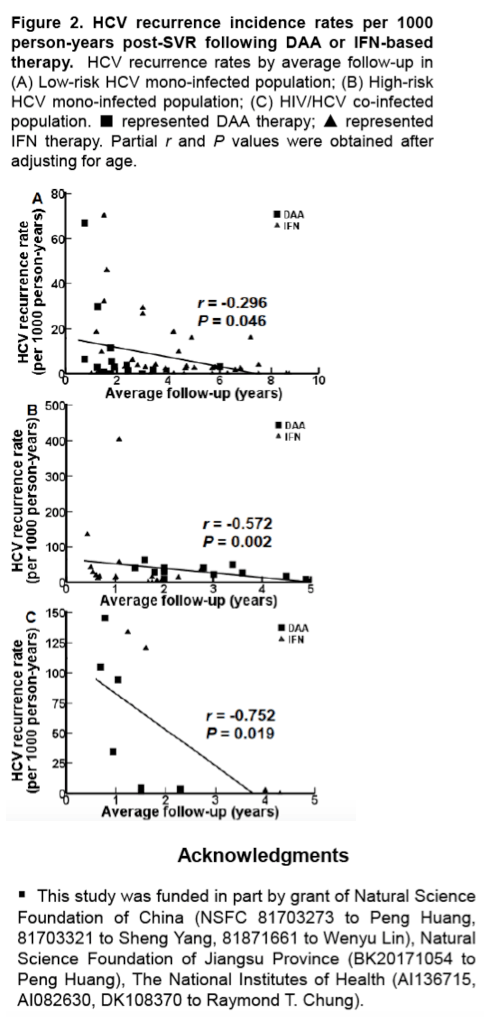
Results: In the 16 studies of HCV mono-infected low-risk patients (n = 16017), the pooled recurrence rate was 0.89/1000 PYFU (95% confidence interval [CI], 0.16-2.03; I2 = 61%), with a corresponding 5-year recurrence risk of 0.44% (95% CI, 0 .08%-1 .01%) . In contrast, in the 17 studies of HCV mono- infected high-risk patients (n = 7559), the pooled recurrence rate was 21 .46/1000 PYFU (95% CI, 10 .54-35 .50; I2 = 86%), with a corresponding 5-year recurrence risk of 10.28% (95% CI, 5 .16%-16 .53%) . In the 8 studies of HIV/HCV co- infected patients (n = 3112), the pooled recurrence rate was 23 .25/1000 PYFU (95% CI, 4 .24-53 .39; I2 = 88%), leading to a 5-year recurrence risk of 11.10% (95% CI, 2 .10%-23 .99%). The higher pooled estimates of recurrence in the high-risk HCV mono-infected and HIV/HCV co-infected populations were predominantly driven by an increase in reinfection rather than late relapse.
Conclusion: HCV recurrence risk after achieving SVR with all oral DAA therapy is low . However, we confirmed that high-risk HCV mono-infected and HIV/ HCV co-infected patients had higher rates of HCV recurrence compared to low-risk HCV mono-infected patients .
|
| |
|
 |
 |
|
|