 |
 |
 |
| |
Risk of severe acute liver injury among initiators of potentially hepatotoxic drugs.
|
| |
| |
Antimicrobials and Antiarrhythmics Lead in Risk of Severe Acute Liver Injury
AASLD The Liver Meeting Digital Experience, November 13-16, 2020
Mark Mascolini
Among drugs causing severe acute liver injury (ALI) in a big group of US veterans, certain antimicrobials and antiarrhythmics had the highest severe ALI incidence from 2000 through September 2017 [1]. But incidence of drug-related severe ALI varied widely in this large veterans study.
Researchers from the University of Pennsylvania and other centers noted that drug-induced acute liver injury explains 10% of all adverse drug reactions. Because such reactions can lead to acute liver failure, transplantation, and death, they are a key reason for withdrawal of marketed agents. But because reports of liver toxicity rarely include drug exposure durations, establishing severe ALI incidence is difficult.
To address that problem, the researchers aimed to calculate severe ALI incidence regardless of cause for drugs with a track record of liver injury. They created a series of retrospective cohorts in the Veterans Health Administration (VHA) Birth Cohort, which enrolls veterans born in 1945-1965 and in care at VHA centers from January 2000 through September 2017.
The investigators used an established hepatotoxicity classification system [2] based on liver injury rates recorded on the US LiverTox website (https://livertox.nlm.nih.gov). The analysis involved cohorts of new users of 45 drugs that had at least 50 reports of liver injury. Researchers included veterans who had at least 1 outpatient prescription for one of the 48 drugs in 2000-2007 and had at least 180 days of VHA care before starting the drug. They excluded anyone with severe ALI within the 180 before starting the drug or who used warfarin. After excluding drugs with fewer than 5 severe ALI events, the researchers had 32 drugs to assess.
The research team defined severe ALI as an inpatient or outpatient international normalized ratio (INR, measuring prothrombin time) at or above 1.5 and total bilirubin more than 2 times the upper limit of normal within 30 days of each other. Follow-up ran from the date a person started the drug to severe ALI (the primary outcome) or stopping the drug, starting warfarin or a direct oral anticoagulant, death, 12 months after the starting date, or September 30, 2017.
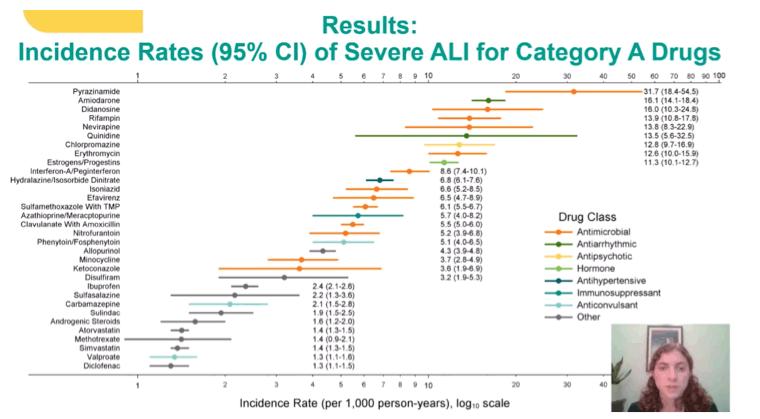
The 9 drugs responsible for the greatest severe ALI incidence in 197,930 veterans who used one of those drugs for the first time included 5 antimicrobials, 2 antiarrhythmics, 1 antipsychotic, and 1 hormone replacement agent:
Pyrazinamide (antimicrobial): 31.7 per 1000 person-years, 95% confidence interval (CI) 18.4 to 54.5
Amiodarone (antiarrhythmic): 16.1 per 1000, 95% CI 14.1 to 18.4
Didanosine* (antimicrobial): 16.0 per 1000, 95% CI 10.3 to 24.8
Rifampin (antimicrobial): 13.9 per 1000, 95% CI 10.8 to 17.8
Nevirapine* (antimicrobial): 13.8 per 1000, 95% CI 8.3 to 22.9
Quinidine (antiarrhythmic): 13.5 per 1000, 95% CI 5.6 to 32.5
Chlorpromazine (antipsychotic): 12.8 per 1000, 95% CI 9.7 to 16.9
Erythromycin (antimicrobial): 12.6 per 1000, 95% CI 10.0 to 15.9
Estrogen/progestin (hormone replacement): 11.3 per 1000, 95% CI 10.1 to 12.7
*Rarely used first-generation anti-HIV drugs.
Other drugs that made the list were interferon/pegylated interferon, hydralazine/isosorbide dinitrate, isoniazid, efavirenz, sulfamethoxazole with trimethoprim, azathioprine/mercaptopurine, clavulanate with amoxicillin, nitrofurantoin, phenytoin/fosphenytoin, allopurinol, minocycline, ketoconazole, disulfiram, ibuprofen, sulfasalazine, carbamazepine, sulindac, androgenic steroids, atorvastatin, methotrexate, simvastatin, valproate, and diclofenac.
The researchers cautioned that severe ALI events counted in this analysis could have been caused by alcohol or liver disease progression, they did not account for combinations of hepatotoxic drugs, and the veterans population they studied consisted mostly of men, often with more comorbidities than the general population.
The authors concluded that, although they focused on hepatotoxic drugs with at least 50 reports of ALI, severe ALI rates varied widely among people starting these medications. Calculating severe ALI rates in this way, they proposed, can help clinicians, pharmacologists, and administrators focus on the need for closer hepatotoxicity monitoring of frequently used drugs.
References
1. Mezochow A, Torgersen J, Newcomb CW, et al. Risk of severe acute liver injury among initiators of potentially hepatotoxic drugs. AASLD The Liver Meeting Digital Experience, November 13-16, 2020. Abstract 119
.
2. Björnsson ES, Hoofnagle JH. Categorization of drugs implicated in causing liver injury: Critical assessment based on published case reports. Hepatology. 2016;63:590-603. doi: 10.1002/hep.28323. https://aasldpubs.onlinelibrary.wiley.com/doi/full/10.1002/hep.28323
|
| |
|
 |
 |
|
|