 |
 |
 |
| |
A 52-WEEK MULTI-CENTER DOUBLE-BLIND RANDOMIZED PHASE 2 STUDY OF SELADELPAR, A POTENT AND SELECTIVE PEROXISOME PROLIFERATOR-ACTIVATED RECEPTOR DELTA (PPAR-DELTA) AGONIST, IN PATIENTS WITH NONALCOHOLIC STEATOHEPATITIS (NASH)
|
| |
| |
AASLD 2020
Nov 11-16
Stephen A Harrison1, Nadege T Gunn1, Arun Khazanchi2, Cynthia D. Guy3, Elizabeth M. Brunt4, Sam Moussa5, Seth J Baum6, Juan P. Frias7, James F. Trotter8, Donald Lazas9, Anita Kohli10, Bradley Vander Veen11, Ziad H Younes12, Ann C. Moore10, Jason L. Huffman13, John Poulos14, Pol Boudes15, Stephen Rossi15, Yun-Jung Choi15, Alexandra Steinberg15, Sujal Shah15, Klara Dickinson15 and Charles McWherter15, (1)Pinnacle Clinical Research, (2)Florida Research Institute, (3)Pathology, Duke University, (4)Washington University in St. Louis, (5)Medical, Adobe Gastroenterology, (6)Excel Medical Clinical Trials, (7)National Research Institute, (8) Texas Digestive Disease Consultants, (9)Digestive Health Research/Objectivegi, Nashville, TN, USA, (10)Arizona Liver Health, (11)Premier Research Group, (12)Gastro One, (13) Gastrointestinal Associates, (14)Cumberland Research
Associates, (15)Cymabay Therapeutics
Background: Seladelpar is a potent PPAR-delta agonist. A study in NASH patients was conducted to confirm the reductions in liver fat, liver enzymes, NASH and fibrosis observed in pre-clinical models.
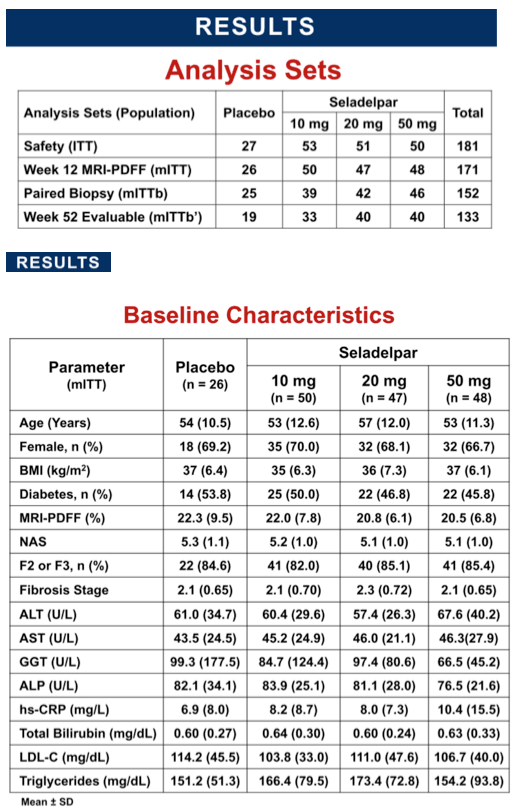
Methods: Eligible patients had liver fat content (LFC) of ≥10% by MRI-PDFF and biopsy- confirmed NASH with a NAFLD activity score (NAS) ≥4 and fibrosis stage F1-F3. Patients were randomized 2:2:2:1 to seladelpar 10, 20 or 50 mg or placebo (pbo) orally once daily for 52 wks. Stratification was based on diabetic status and F1 vs F2-F3. The primary outcome was change in LFC at Wk 12 and the study continued blinded to Wk 52 for follow-up biopsy . Serial measures included liver enzymes and non-invasive imaging. All biopsies were centrally read for screening (1 pathologist) and Wk 52 (1 or 2 pathologists) and were blinded to treatment but not to study timepoint.
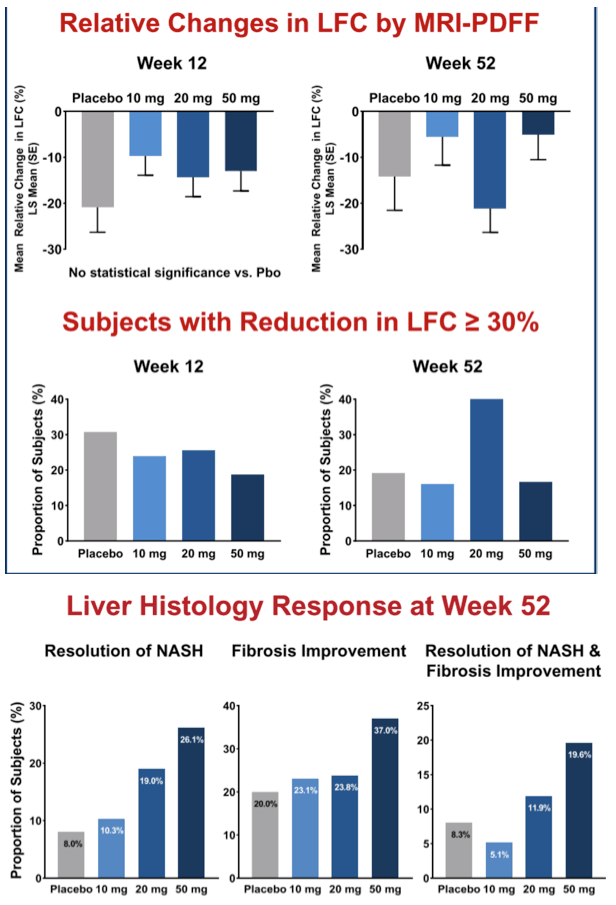
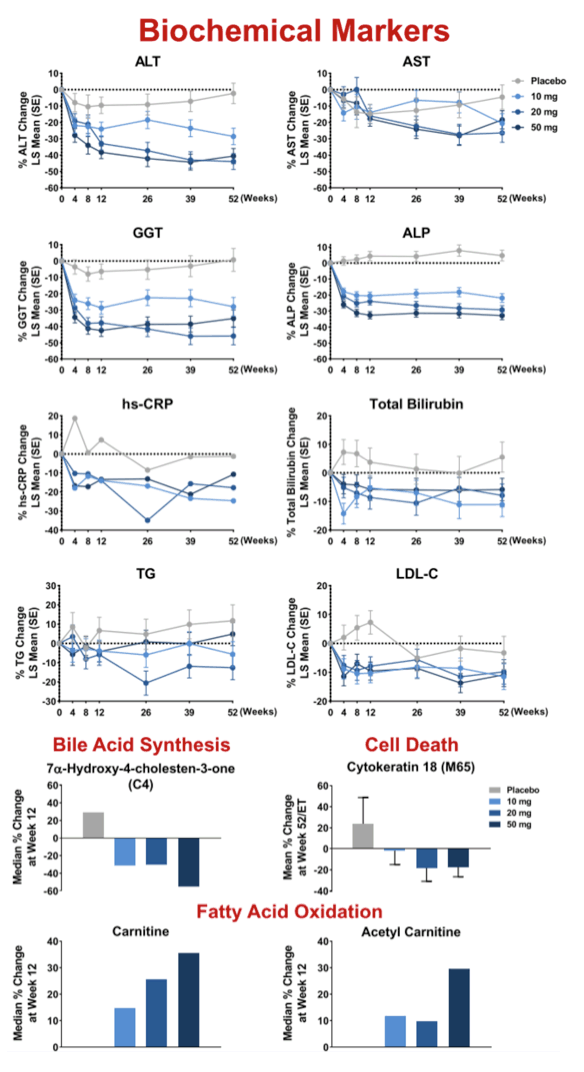
Results: 181 patients (14 US sites) were randomized with mean BMI 35.9 kg/m2, LFC 21 .5%, NAS 5.2, and with 83% F2/F3 and 49% diabetic. Baseline (BL) mean liver enzymes were ALT 62.2 U/L, AST 46.1 U/L, and GGT 86.6 U/L. 152 patients (84%) had Wk 52/early termination (ET) biopsy. The study was terminated when Wk 52/ET biopsy revealed "unexpected" liver pathology (42/152); independent review found most pathology present at BL and not caused by seladelpar (reported elsewhere). LFC in seladelpar groups declined from BL at Wks 12 and 52 (-8 to -24%) but they were not different from pbo (-18 to -21 %). Serum ALT and GGT decreased significantly in a dose-ordered manner up to -41% and -35%, respectively.
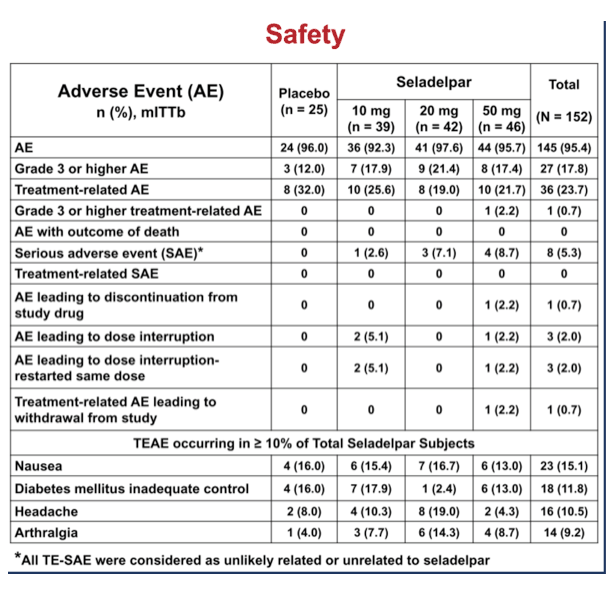
Secondary endpoint analysis revealed NASH resolution with no increase in fibrosis in 2 (8%), 4 (10%), 8 (19%) and 12 (26%) patients in the pbo, 10, 20 and 50 mg groups, respectively (NS). Decrease in fibrosis with no worsening in NASH was seen in 5 (20%), 9 (23%), 10 (24%), and 17 (37%) (NS) patients in these same groups. NASH resolution and fibrosis improvement was seen in 2 (8%), 2 (5%), 5 (12%) and 9 (20%), respectively. AEs were balanced. There were 9 SAEs, none related to seladelpar; 7 non-serious TEAEs led to discontinuation.
Conclusion: Seladelpar decreased LFC, but with the large pbo effect, the primary 12-wk endpoint was not met. Seladelpar treatment led to significant reductions in key liver enzymes and while not significant vs pbo, dose dependent improvements in liver pathology, including decreases in fibrosis, were also observed. Seladelpar appears to be safe and was well tolerated.

|
| |
|
 |
 |
|
|