 |
 |
 |
| |
SEMAGLUTIDE TREATMENT IN SUBJECTS WITH NAFLD: EFFECTS ASSESSED BY MAGNETIC RESONANCE ELASTOGRAPHY AND MAGNETIC RESONANCE IMAGING PROTON DENSITY FAT FRACTION
|
| |
| |
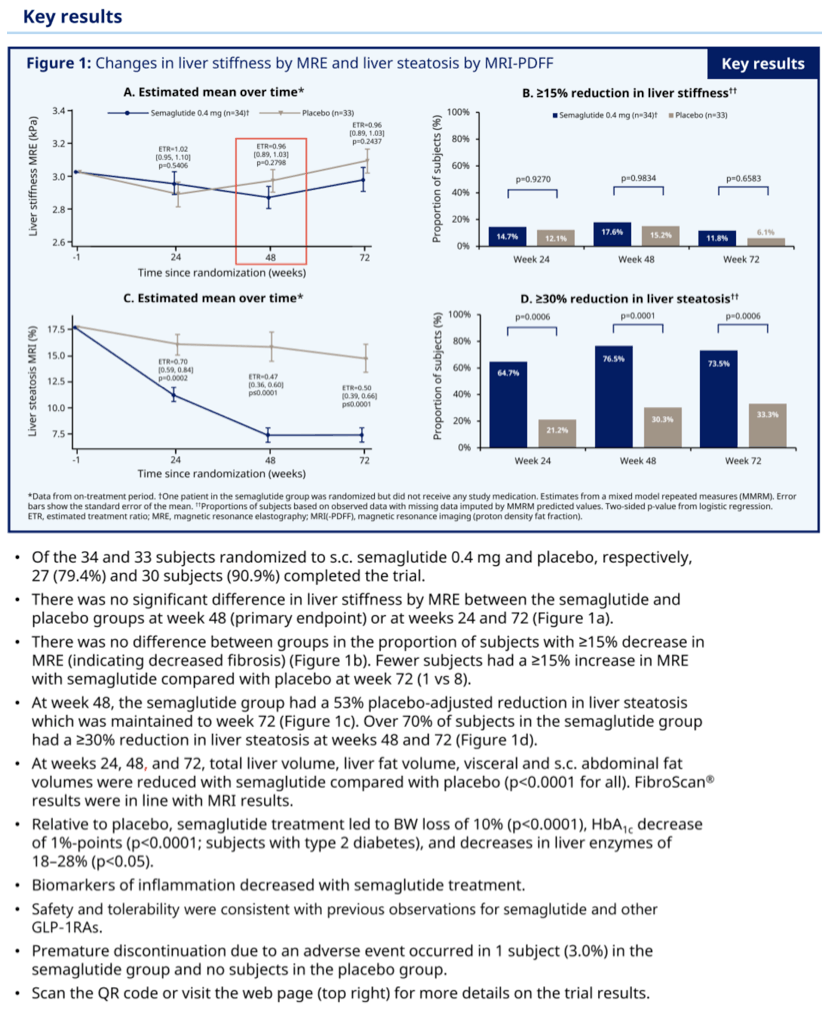
At weeks 24, 48 and 72, total liver volume, liver fat volume, visceral and sc abdominal fat volumes were reduced with sema vs PBO (all p<0 .0001).
AASLD 2020 Nov 11-16
Anne Flint1, Grit Andersen2, Paul Hockings3, Lars Johansson3, Anni Morsing1, Mads Sundby Palle1, Thomas J Vogl4 and Leona Plum-Moerschel2, (1)Novo Nordisk a/S, (2)Profil, (3) Antaros Medical, (4)Goethe-Universitat Frankfurt
Background: Non-alcoholic fatty liver disease (NAFLD) is thought to be the liver manifestation of metabolic syndrome . Among glucagon-like peptide-1 receptor agonists (GLP- 1RAs), semaglutide (sema) has the most pronounced effect on body weight (BW) . This study investigated the effect of sema on NAFLD, using non-invasive magnetic resonance imaging (MRI) methods and exploratory biomarkers.
Methods: 67 subjects with NAFLD (MRI proton density fat fraction [MRI-PDFF] ≥10%) and increased liver stiffness (magnetic resonance elastography [MRE] 2 .50-4 .63 kPa) were randomly assigned 1:1 to subcutaneous (sc) sema 0 .4 mg/day or placebo (PBO) for 72 weeks . Liver stiffness and liver fat content were measured by MRE and MRI-PDFF, respectively, after 24, 48 and 72 weeks of treatment . Liver enzymes (ALT, AST, GGT) and exploratory blood-based biomarkers for non-alcoholic steatohepatitis (NASH) were measured along with FibroScan, visceral and sc abdominal fat, BW and metabolic parameters.
Results: At 48 weeks, there was no difference in liver stiffness (primary endpoint) between the sema and PBO groups (MRE, estimated treatment ratio (ETR) [95%CI]: 0 .96 [0 .89-1 .03], p=0 .2798), and no difference in liver stiffness was observed at week 72. The number of subjects with MRE values increasing ≥15%, indicating increased fibrosis, was lower for sema vs PBO at week 48 (1 vs 8, respectively). At week 48, the sema group had a 58% reduction in liver fat vs PBO (MRI-PDFF; ETR [95%CI]: 0 .47 [0 .36-0 .60], p<0 .0001); this reduction was maintained through week 72. Over 70% of subjects in the sema group had a reduction of ≥30% in liver fat. At weeks 24, 48 and 72, total liver volume, liver fat volume, visceral and sc abdominal fat volumes were reduced with sema vs PBO (all p<0 .0001). FibroScan results were in line with MRI results. Relative to PBO, sema treatment led to BW loss of 10% (p<0 .0001), HbA1c decrease of 1%-points (p<0 .0001; subjects with type 2 diabetes) and decreases in liver enzymes of 18-28% (p<0.05). Biomarkers of inflammation decreased with sema treatment . Safety and tolerability were consistent with previous observations for sema and other GLP-1RAs.
Conclusion: Sema significantly reduced liver fat, which together with other findings, suggests a positive impact on disease activity and metabolic profile in a NAFLD population. After sema treatment, no apparent improvement in liver stiffness was observed, but fewer subjects had substantial increases in this marker of fibrosis.


|
| |
|
 |
 |
|
|