 |
 |
 |
| |
Real World Single Centre Experience on the Efficacy of Stopping Long Term Nucleos(t)ide Analog Therapy in
Patients with Chronic Hepatitis B
|
| |
| |
AASLD 2020 Nov 11-16 virtual
H Azhari, AD Frolkis, AA Shaheen, H Israelson, J Pinto, SE
Congly, MA Borman, AA Aspinall, LM Stinton, MG Swain,
KW Burak, SS Lee, MD Sadler and CS Coffin

abstract
Background: Nucleos(t)ide analogs (NA) suppress hepatitis B virus (HBV) replication but have minimal effect on the intrahepatic HBV cccDNA template and rarely lead to HBsAg loss, requiring prolonged therapy . Although expert guidelines recommend that HBeAg (+) patients may stop NA after achieving HBeAg seroconversion and consolidation therapy, many relapse with treatment cessation . In HBeAg (-) chronic hepatitis B (CHB) some studies report that NAs can be safely stopped in non-cirrhotic patients with undetectable HBV DNA and quantitative (q)HBsAg levels <100 IU/mL . This study aimed to determine baseline clinical factors that are associated with off-treatment NA durability.
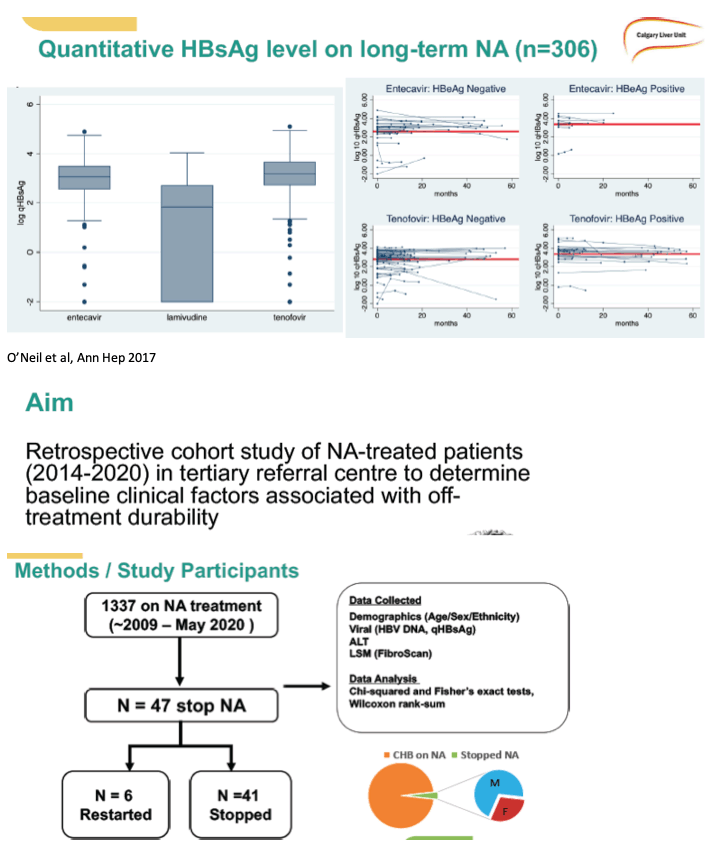
Methods: In this observational cohort study conducted at the Liver Unit of the University of Calgary in Calgary, AB, Canada, we reviewed all NA treated patients who stopped long-term NA therapy based on physician recommendation at our centre . Baseline characteristics including age, sex, ethnicity, HBV infection profile, and liver stiffness by transient elastrography (FibroScan®) were collected . Data after stopping therapy was collected as per standard of care (i .e ., qHBsAg, ALT, HBV DNA) . Categorical variables were compared using Chi-squared and Fisher's exact tests . Continuous variables were compared using Wilcoxon rank-sum tests.
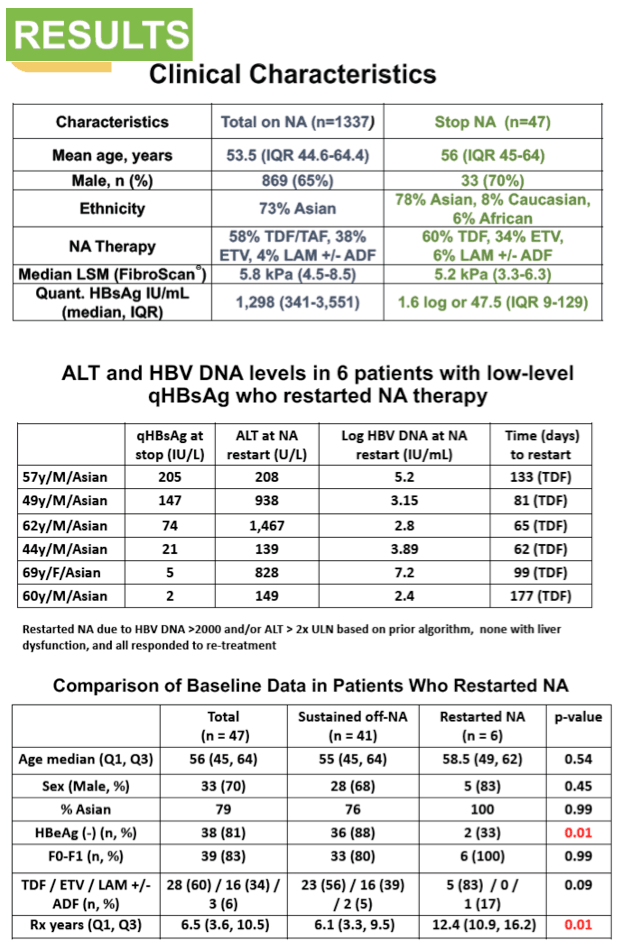
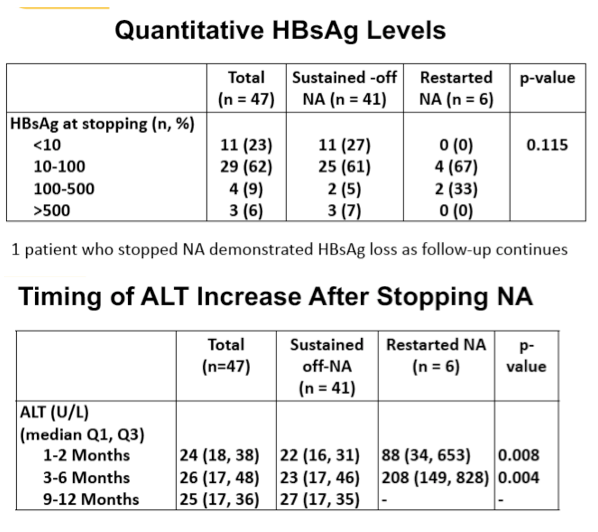
Results: Among 1,337 CHB patients on long-term NA, 47 stopped therapy (29 .8% Female, 78% East Asian, median age 56 years, range 18-74 years), 28/47 were on tenofovir disoproxil fumarate, 16 on entecavir, and 3 on lamivudine (including 2 with adefovir combination). All were HBeAg (-) at the time of NA discontinuation and 46/47 had undetectable HBV DNA. Median liver stiffness was 5 .2 kPa (range 3 .3-6 .6kPa). Six patients (qHBsAg at NA discontinuation 205, 147, 74, 21, 5 .4, 2.3) restarted NA due to a virologic flare defined as HBV DNA >2000IU/mL (median HBV DNA 4,663 IU/mL, range 265 - 152,043,102 IU/mL) and/or biochemical flare defined as ALT >2xULN (ALT 208, 938, 1467, 139, 828, 149 respectively). None developed hepatic dysfunction, and all responded to restarting NA with suppressed HBV DNA. Factors associated with needing to restart NA included baseline HBeAg (+) status pre-treatment (n=3; 50% in the relapse group; n=5; 12% in the non-relapse group; p=0 .004) and longer NA treatment duration (median duration 12 .4 years in the relapse group; 6 .1 years in the non-relapse group, p=0 .011). Age, sex, liver stiffness, NA, ethnicity, and qHBsAg level at stopping were not associated with relapse risk. Our data suggests that patients who experience a flare up do so within the first 6 months of discontinuing NAs, and are less likely to have a flare up once this period has passed safely.
Conclusion: Stopping long- term NA is feasible in HBeAg negative CHB with qHBsAg <100IU/mL, however severe ALT flares can occur warranting close follow-up . The majority of biochemical and virological flares occur within the first 6 months of treatment cessation.

|
| |
|
 |
 |
|
|