| |
WHO Report: HIV+ COVID Responses
|
| |
| |
Download the PDF here
pdf attached of WHO SLIDES
WHO SOLIDARITY TRIAL
Adults (age ≥18 years) recently hospitalised, or already in hospital, with confirmed COVID-19 and, in the view of the responsible doctor, no contra-indication to any of the study treatments will be randomly allocated between
• Local standard of care,
OR local standard of care plus one of
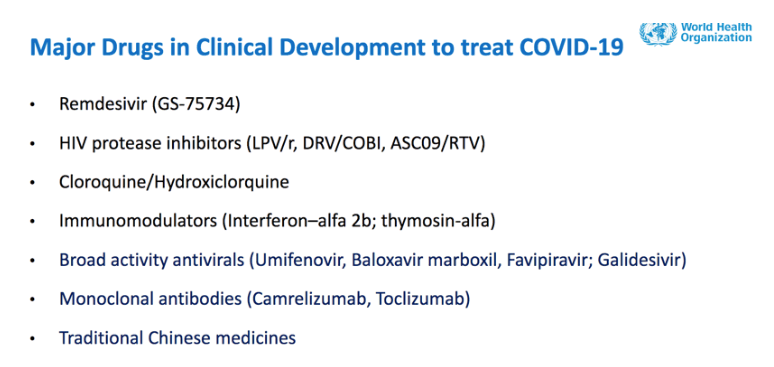
• Remdesivir
• Chloroquine or Hydroxychloroquine
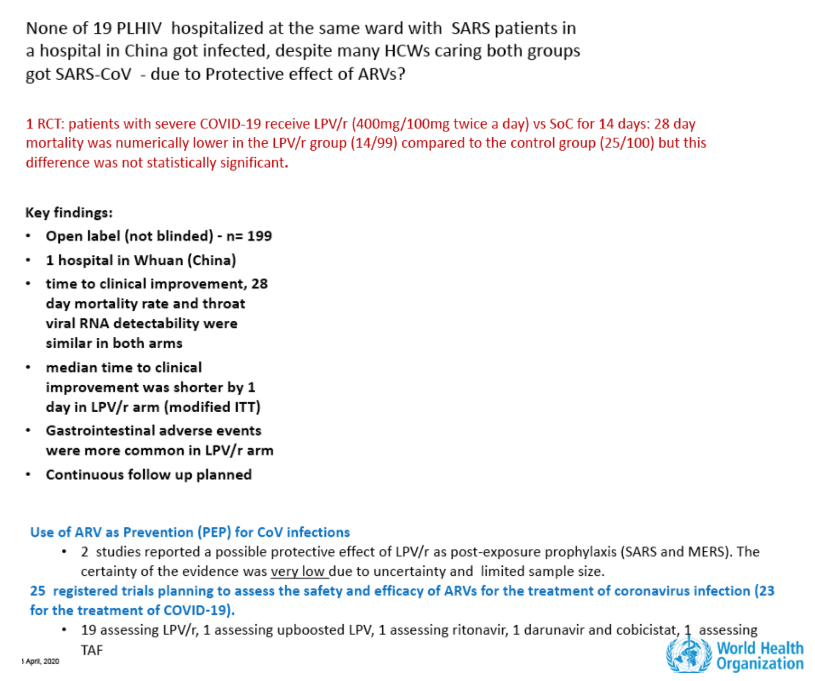
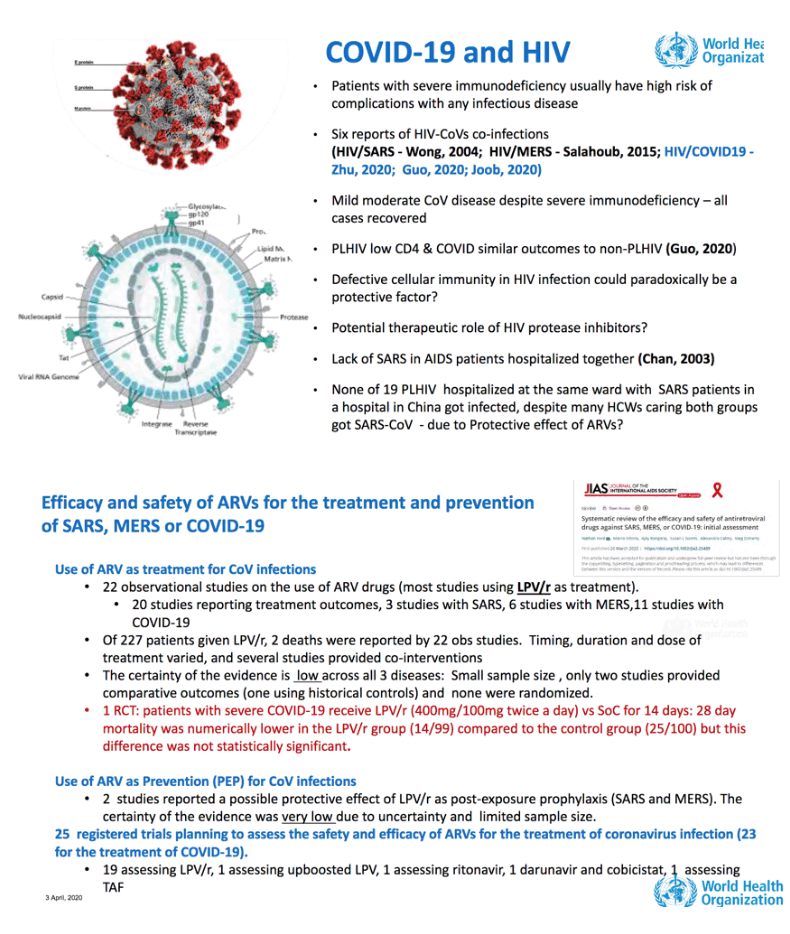
• Lopinavir with Ritonavir
• Lopinavir with Ritonavir plus Interferon beta-1a.
Underlying conditions recorded are: diabetes, heart disease, chronic lung disease, chronic liver disease and asthma, extending to HIV and tuberculosis in the African region.
Severity of illness at entry is determined by recording: shortness of breath, being given oxygen, already on a ventilator, and, if lungs imaged, major bilateral abnormality.
IAS COVID WEBINAR:
https://www.iasociety.org/HIV-Programmes/Cross-cutting-issues/COVID-19-and-HIV-Webinars
WEBINAR:
https://www.iasociety.org/HIV-Programmes/Cross-cutting-issues/COVID-19-and-HIV-Webinars


|
|
| |
| |
|
|
|