 |
 |
 |
| |
Despite Evidence of Benefit in NASH, GLP1a and SGLT2i Use Low in US
|
| |
| |
EASL 2020, Digital International Liver Congress, August 27-29, 2020
Download the PDF here
Mark Mascolini
Despite evidence that GLP-1 receptor agonists (GLP1a) and SGLT-2 inhibitors (SGLT2i) have potential benefits in people with nonalcoholic steatohepatitis (NASH) or at risk for NASH [1,2], in US practice use of these agents remains low, between 4% and 7% in people with nonalcoholic fatty liver and type 2 diabetes, and even lower in fatty liver patients without diabetes [3].
Research shows that GLP1a promote histologic resolution of NASH without making fibrosis worse, noted multicenter US collaborators who conducted this study. SGLT2i therapy lowers weight, ALT, and FIB-4. Both types of agents are licensed for treatment of type 2 diabetes. A US research team conducted this analysis to determine how often diabetic and nondiabetic people with or at risk for NASH use these agents.
The researchers culled data from a national proprietary electronic database containing demographic, clinical, and pharmaceutical information from academic, hospital, and community care centers across the United States. They limited the sample to adults with ICD-determined NASH or nonalcoholic fatty liver disease (NAFLD) or with a NASH/NAFLD risk profile: age over 50, ALT over 30 U/L, without viral hepatitis or alcohol abuse, and with either body mass index (BMI) above 30 kg/m2 (obese) or type 2 diabetes. The research team set the index date as the first measurable FIB-4 between July 2015 and June 2017. Every participant had at least 1 year of clinical history before the index date and at least 2 years of follow-up or follow-up to death.
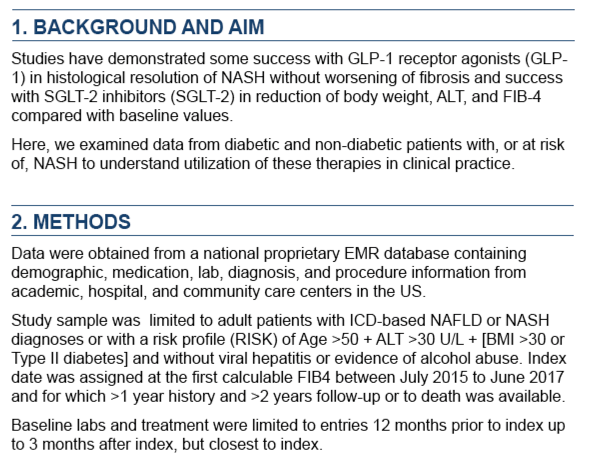
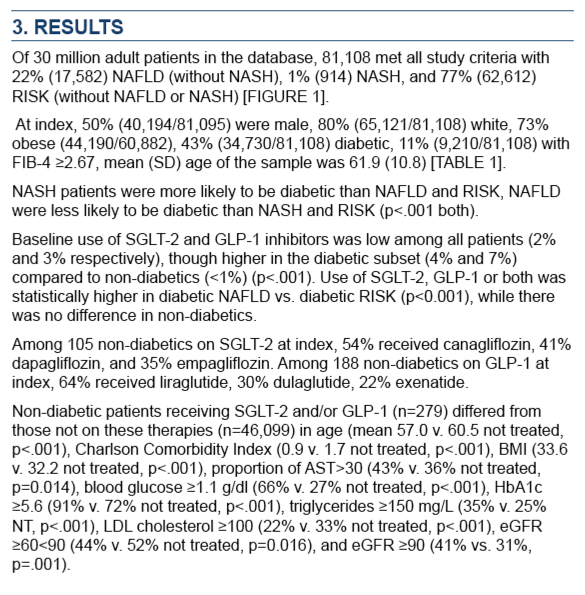
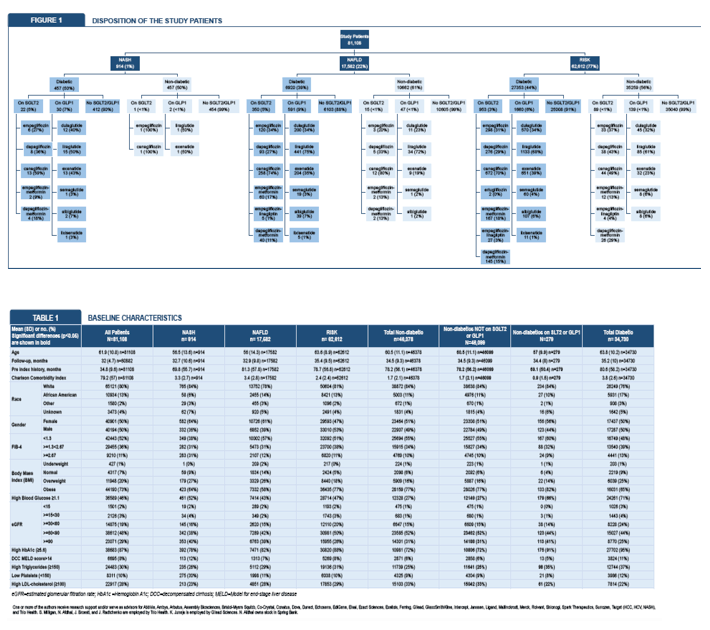
Among 30 million adults in the electronic database, 81,108 met all study criteria. While 22% had NAFLD without NASH, 1% had NASH, and 77% met risk criteria without a diagnosis of NAFLD or NASH. At the index date, 50% of the cohort were men, 80% white, 73% obese, 43% diabetic, and 11% with FIB-4 at or above 2.67. Age averaged 61.9 years.
At the index data, only 2% of the cohort used SGLT2i and 3% used GLP1a. Respective use rates were higher in people with diabetes, 4% and 7%, compared with nondiabetics (below 1%) (P < 0.001). Use of SGLT2i and/or GLP1a was significantly higher in people with diabetic NAFLD than in those in the diabetic at-risk group (P < 0.001). Use of these agents did not differ between nondiabetics with NAFLD or NASH and nondiabetics in the at-risk group.
Among the 105 nondiabetics taking SGLT2i at the index date, the most frequently used drugs were canagliflozin (54%), dapagliflozin (41%), and empagliflozin (35%). Among 188 nondiabetics taking GLP1a at the index date, the most-used agents were liraglutide (64%), dulaglutide (30%), and exenatide (22%).
The 279 nondiabetics receiving SGLT2i and/or GLP1a differed significantly from the 46,099 nondiabetics not using those agents in age (average 57.0 vs 60.5, P < 0.001), Charlson Comorbidity Index (0.9 vs 1.7, P < 0.001), BMI (33.6 vs 32.2 kg/m2, P < 0.001), and proportions with AST above 30 U/L (43% vs 36%, P = 0.014), blood glucose at or above 1.1 g/dL (66% vs 27%, P < 0.001), HbA1c at or above 5.6 (91% vs 72%, P < 0.001), triglycerides at or above 150 mg/L (35% vs 25%, P < 0.001), LDL cholesterol at or above 100 mg/dL (22% vs 33%, P < 0.001), eGFR between 60 and 90 mL/min (44% vs 52%, P = 0.016), and eGFR at or above 90 mL/min (41% vs 31%, P = 0.001).
Despite evidence of the potential benefits of SGLT2i and GLP1a in people with or at risk for NASH, the researchers conclude, "real-world use of these therapies" was low in people with nonalcoholic fatty liver and type 2 diabetes (4% to 7%), even though insurance often covers SGLT2i and GLP1a in diabetics. Fewer than 1% of people with or at risk for NASH but without diabetes used SGLT2i or GLP1a. In this group, use of these agents was higher in younger people, those with fewer comorbidities, and those with lab measures similar to people with type 2 diabetes.
References
1. Lv X, Dong Y, Hu L, Lu F, Zhou C, Qin S. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) for the management of nonalcoholic fatty liver disease (NAFLD): A systematic review. Endocrinol Diabetes Metab. 2020;3:e00163. doi: 10.1002/edm2.163.
2. Scheen AJ. Beneficial effects of SGLT2 inhibitors on fatty liver in type 2 diabetes: A common comorbidity associated with severe complications. Diabetes Metab. 2019;45:213-223. doi: 10.1016/j.diabet.2019.01.008.
3. Lai M, Broestl J, Juneja K, et al. Use of GLP-1 receptor agonists (GLP1a) and/or SGLT-2 inhibitors (SGLT2i) in populations with NASH or at risk of NASH in US clinical practice. EASL 2020, Digital International Liver Congress, August 27-29, 2020. Abstract FRI107.
-------------------
Use of GLP-1 Receptor Agonists (GLP1a) and/or SGLT-2 Inhibitors (SGLT2i) in
Populations with NASH or at Risk of NASH in US Clinical Practice USA
1Michelle Lai, 2Jeremy Broestl, 3Kavita Juneja, 2Scott Milligan, 2Janna Radtchenko, 4 5Zobair Younossi, 1Nezam Afdhal
1Beth Israel Deaconess Medical Center (BIDMC), Boston, USA, 2Trio Health Analytics, La Jolla, USA, 3Gilead Sciences, Inc., Foster City, USA, 4Inova Fairfax Hospital, Center for Liver Diseases, Department of Medicine, Falls Church, USA, 5Inova Health System, Betty and Guy Beatty Center for Integrated Research, Falls Church, USA

1Michelle Lai, 2Jeremy Broestl, 3Kavita Juneja, 2Scott Milligan, 2Janna Radtchenko, 4 5Zobair Younossi, 1Nezam Afdhal
1Beth Israel Deaconess Medical Center (BIDMC), Boston, USA, 2Trio Health Analytics, La Jolla, USA, 3Gilead Sciences, Inc., Foster City, USA, 4Inova Fairfax Hospital, Center for Liver Diseases, Department of Medicine, Falls Church, USA, 5Inova Health System, Betty and Guy Beatty Center for Integrated Research, Falls Church, USA

|
| |
|
 |
 |
|
|