 |
 |
 |
| |
SAFETY AND EFFICACY OF CABOTEGRAVIR + RILPIVIRINE LONG-ACTING WITH AND WITHOUT ORAL LEAD-IN: FLAIR WEEK 124 RESULTS
|
| |
| |
Glasgow 2020 Oct 5-8 virtual
Reported by Jules Levin
Ronald D'Amico1, Chloe Orkin2, Enrique Bernal Morell3, Darrell H. S. Tan4, Harold Katner5, Yashna Singh6, Hans-Jürgen Stellbrink7, Elena Belonosova8, Rebecca DeMoor9, Sandy Griffith1, Shanker Thiagarajah9, Rodica Van Solingen-Ristea10, Herta Crauwels10, Susan L. Ford11, Parul Patel1, Amy Cutrell1, Kimberly Y. Smith1, Kati Vandermeulen10, David A. Margolis1, Marty St. Clair1, William R. Spreen1
1ViiV Healthcare, Research Triangle Park, NC, United States; 2Queen Mary University, London, United Kingdom; 3Hospital General Universitario Reina Sofía, Murcia, Spain; 4Division of Infectious Diseases, St. Michael's Hospital, Toronto, Canada; 5Mercer University Medical School, Macon, GA, United States; 6Desmond Tutu HIV Foundation, Cape Town, South Africa; 7ICH Study Center, Hamburg, Germany; 8Orel Regional Center for AIDS, Orel, Russia; 9GlaxoSmithKline, London, United Kingdom; 10Janssen Research and Development, Beerse, Belgium; 11GlaxoSmithKline, Research Triangle Park, NC, United States
1. Orkin C, et al. N Engl J Med. 2020;382(12):1124-1135.
2. Orkin C, et al. CROI 2020; Boston, Massachusetts; March 8-11, 2020; Poster 0482.
3. Swindells S, et al. N Engl J Med. 2020;382(12):1112-1123.
4. Overton ET, et al. Lancet. Accepted 2020.
CAB, cabotegravir; CVF, confirmed virologic failure; DTI, direct to inject; ISR, injection site reaction; LA, long-acting; OLI, oral lead-in; RPV, rilpivirine.


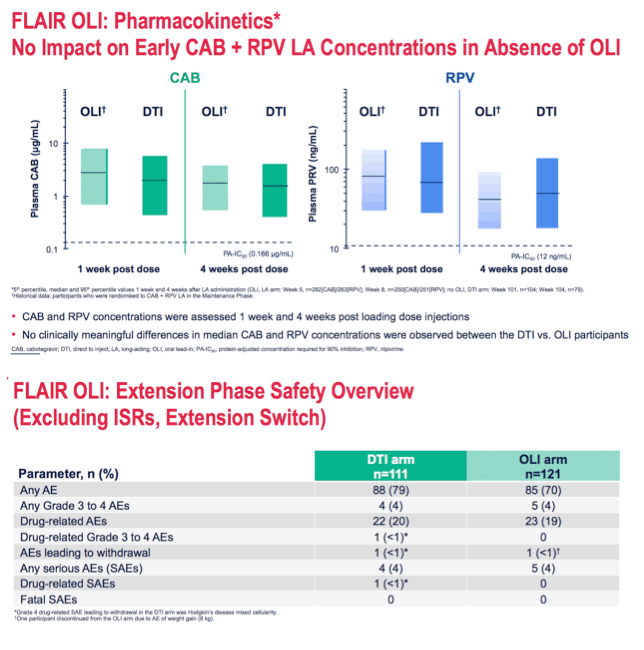
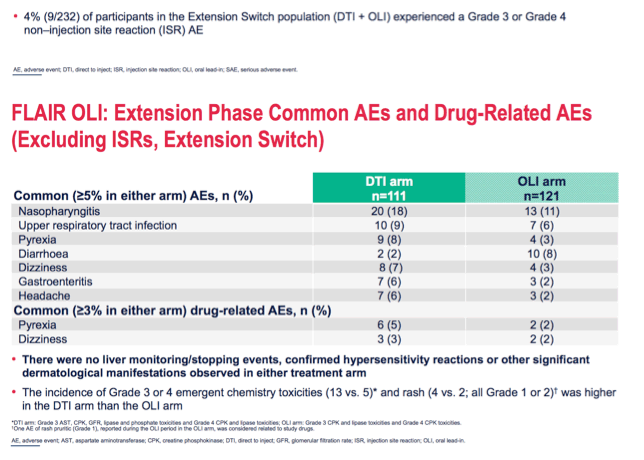
*DTI arm: Grade 3 AST, CPK, GFR, lipase and phosphate toxicities and Grade 4 CPK and lipase toxicities; OLI arm: Grade 3 CPK and lipase toxicities and Grade 4 CPK toxicities.
One AE of rash pruritic (Grade 1), reported during the OLI period in the OLI arm, was considered related to study drugs.
AE, adverse event; AST, aspartate aminotransferase; CPK, creatine phosphokinase; DTI, direct to inject; GFR, glomerular filtration rate; ISR, injection site reaction; OLI, oral lead-in.
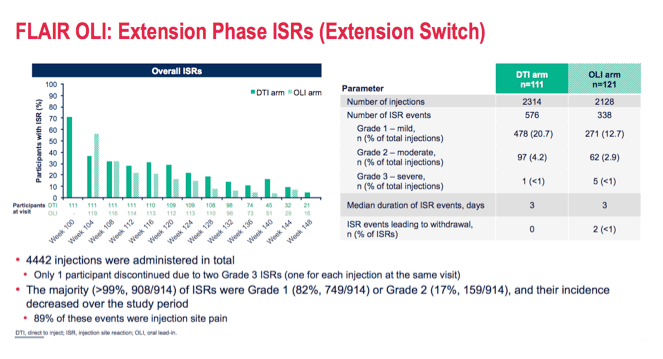
|
| |
|
 |
 |
|
|