 |
 |
 |
| |
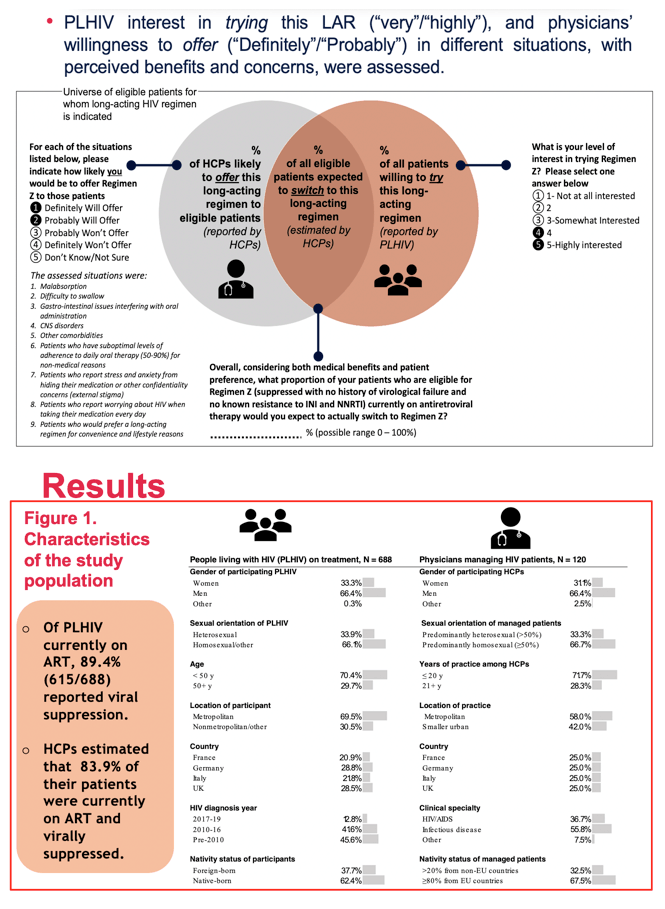
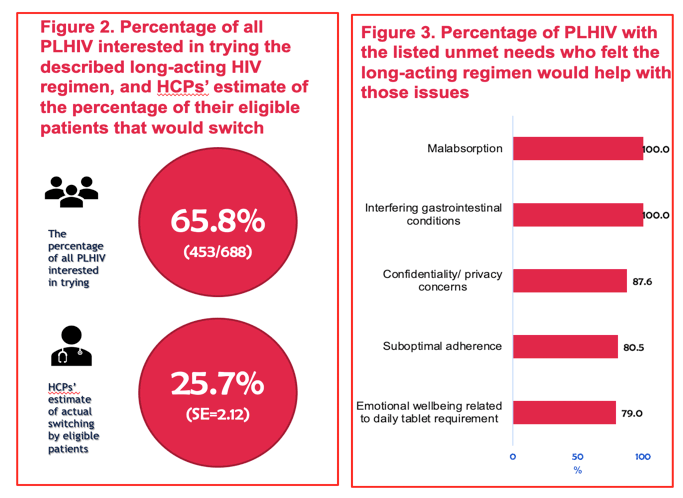
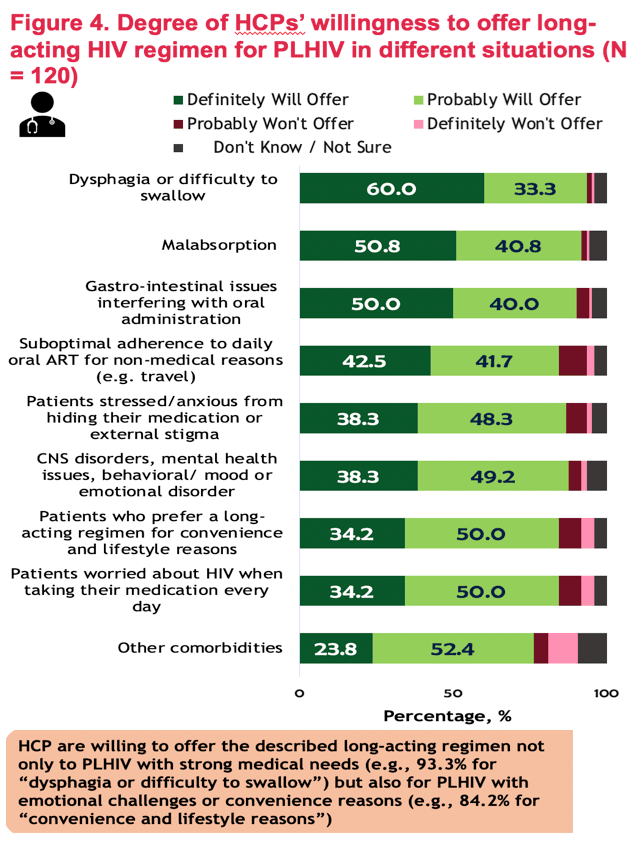
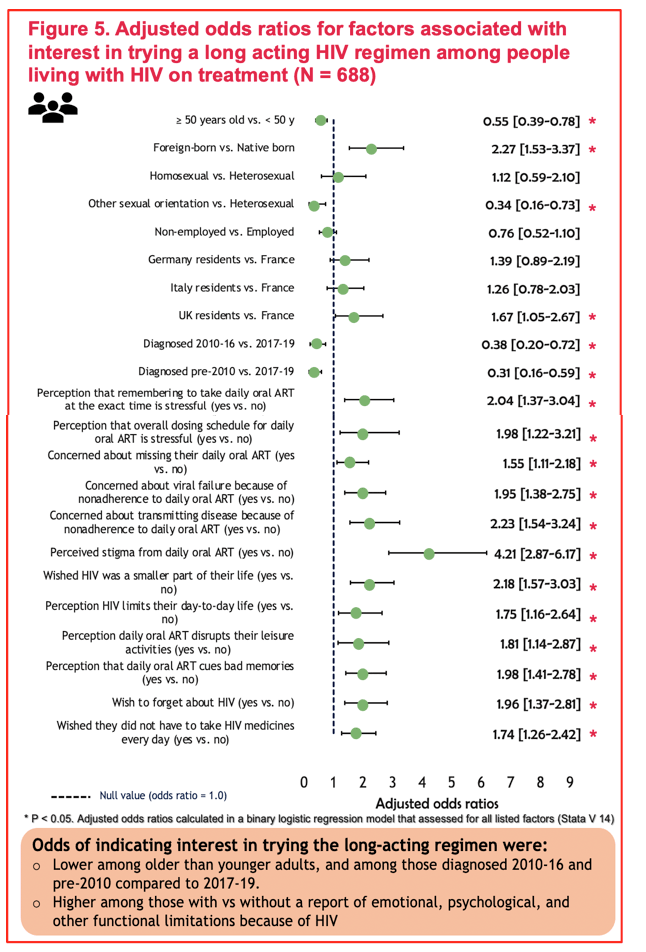
FACTORS ASSOCIATED WITH INTEREST IN A LONG-ACTING HIV REGIMEN: PERSPECTIVES OF PEOPLE LIVING WITH HIV, AND PHYSICIANS IN WESTERN EUROPE
|
| |
| |
Download the PDF here
Glasgow 2020 Oct 5-8 virtual
Reported by Jules Levin
Babatunde Akinwunmi MD, MPH, MMSc1 ; Daniel Buchenberger MS 2; Jenny Scherzer, MSc3; Martina Bode, MSc3;
Paolo Rizzini, MD4; Fabio Vecchio, MSc4; Laetitia Roustand, MSc5; Gaelle Nachbaur5; Laurent Finkielsztejn6
1Zatum LLC, USA; 2Ipsos Insights LLC, New York, NY, USA; 3ViiV Healthcare, Munich, Germany; 4ViiV Healthcare, Verona, Italy; 5GlaxoSmithKline, Rueil Malmaison, France; 6ViiV Healthcare, Rueil Malmaison, France; 7ViiV Healthcare, Brentford Middlesex, UK Presenting author: Nicolas Van de Velde; nicolas.x.van-de-velde@gsk.com

References: 1. de Los Rios P, Okoli C, Punekar Y, et al. Prevalence, determinants, and impact of suboptimal adherence to HIV medication in 25 countries [published online ahead of print, 2020 Jun 25]. Prev Med. 2020;139:106182. doi:10.1016/j.ypmed.2020.1061822. 2. Clark L, Karki C, Noone J, et al. Quantifying people living with HIV who would benefit from an alternative to daily oral therapy: Perspectives from HIV physicians and people living with HIV. Population Medicine. 2020 (In press). 3. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390(10101):1499-1510. doi:10.1016/S0140-6736(17)31917-7. 4. L Slama, R Porcher, C Chakvetadze, A Cros, F Linard, L Gallardo, JP Viard, S Carillon, JM Molina. Injectable long acting antiretrovirals for HIV treatment or prevention: the ANRS CLAPT study. 2019 British HIV Association Conference. EACS 2019 - Abstract Book. PE30/9. https://doi.org/10.1111/hiv.12814.
|
| |
|
 |
 |
|
|