| |
|
Efficacy of NS5A inhibitors against unusual and potentially difficult-to-treat HCV subtypes commonly found in sub Saharan Africa and South East Asia
|
| |
| |
May 26, 2020 - Dung Nguyen1, David Smith1, Alun Vaughan-Jackson2, Andrea Magri3, STOP-HCV Consortium,
Eleanor Barnes1*, Peter Simmonds1*
1 Nuffield Department of Medicine, University of Oxford, Oxford OX1 3SY, UK
2 Sir William Dunn School of Pathology, University of Oxford, OX1 3RE
3 Nuffield Department of Medicine, University of Oxford, Oxford OX3 7FZ, UK
Download the PDF here
Highlights
• The "unusual" HCV subtypes 1l, 3b, 3g, 4r, 6u and 6v show variable levels of resistance to all NS5A inhibitors in-vitro.
• Pibrentasvir shows the greatest level of activity against all the unusual subtypes.
• HCV subtypes 3b and 3g are resistant to all NS5A inhibitors other than pibrentasvir in in-vitro testing.
• Daclatasvir and ledipasvir are ineffective against subtypes 6u and 6v in in-vitro testing.
• The presence of the resistance associated substitutions Q30R in 1l, and L31M or Y93H in 4r confers resistance to ledipasvir.
Abstract
Background Aims
The efficacy of NS5A inhibitors against several less common subtypes of hepatitis C virus (HCV) is poorly characterised. Some subtypes including 3b, 3g, 6u and 6v commonly harbour amino acid residues as wild type in NS5A that may confer resistance to direct acting antivirals (DAAs) in other common subtypes. Data from patients also suggest that 1l and 4r with amino acid substitutions at positions 28-31 and 93 in NS5A are relatively resistant to DAA therapy.
Methods
In this study, we tested the efficacy of daclatasvir, elbasvir, ledipasvir, pibrentasvir and velpatasvir against these subtypes using the SGR-JFH1 replicon backbone.
Results
NS5A inhibitors showed different levels of efficacy with only pibrentasvir effective against all tested subtypes. Daclatasvir and ledipasvir were ineffective against 6u and 6v (half maximal effective concentration [EC50] values of 239-321 nM) while 3b and 3g were only susceptible to pibrentasvir. Analysis of effects of individual mutations indicated that Q30R in 1l increased the EC50 of ledipasvir by 18 fold, conferring intermediate resistance, while those of L31M and Y93H in 4r induced increases in EC50s of 2100- and 3575-fold (high level resistance).
Conclusion
The high ledipasvir EC50 values of 1l with the Q30R substitution, 4r L31M and 4r Y93H may explain the treatment failure in patients who were infected with these viruses and treated with ledipasvir + sofosbuvir. This study also shows the ineffectiveness of the first generation NS5A inhibitors against 6u and 6v, and confirms the inherent resistance of 3b and 3g to most NS5A inhibitors. Clinical studies to confirm in vivo sensitivity to NS5A inhibitors are urgently needed so that rational, effective treatment strategies may be developed for unusual subtypes.
Introduction
Hepatitis C virus (HCV) infection is a major global health concern, with about 500,000 deaths annually and 71.1 million chronically infected individuals. The recent development of highly effective directly acting antiviral therapy (DAAs) has dramatically changed the treatment of HCV and reduced morbidity and mortality. In real-world treatment using DAA-based regimens, sustained virologic response (SVR) rates of > 90% have been reported. In light of this, the World Health Organisation's has developed a global strategy that aims to eliminate HCV by 2030.
HCV NS5A plays an essential role in the viral life cycle, involved in both viral replication and assembly. It is therefore a key target in all currently recommended HCV combination treatment regimens. However, the presence of resistance associated substitutions (RASs) have been shown to reduce the efficacy of NS5A inhibitors in both clinical trials and in vitro studies. RASs to NS5A inhibitors have also been shown to be present more commonly than RASs to protease inhibitors and NS5B inhibitors at treatment baseline, and also to persist for longer when they are selected in response to therapy associated with treatment failure.
HCV is highly diverse with more than 100 subtypes grouped into 8 genotypes. Genotypes 1 and 3 are the most prevalent genotypes worldwide. Genotype 4 is most commonly detected in the Middle East and sub-Saharan African while genotype 6 is mainly found in Southeast Asia. The prevalence of genotypes 4 and 6 is increasing in North America and Europe due to migration. Genotype 5 accounts for < 1% of all HCV cases and is mainly reported from Southern and Eastern sub-Saharan Africa. Genotype 3 was considered the most difficult-to-treat one when DAAs were first licensed (reviewed in 17). However, more recent clinical trial data using the combination of glecaprevir and pibrentasvir or velpatasvir and sofosbuvir have now achieved SVR rates of greater than 95% in patients infected with genotypes 1 through 6. Using an infectious culture system, Gottwein et al. tested the efficacy of NS5A inhibitors against the major subtypes and demonstrated consistently high activity of pibrentasvir and velpatasvir against genotype 1-7 infectious clones.
Many clinical trials have failed to identify subtypes of the common genotypes in the study groups or have not included patients with unusual subtypes. Consequently, the true effectiveness of the full repertoire of NS5A inhibitors now used in clinical practice against many HCV variants is not known. A failure to identify subtypes is of particular concern since there is substantial variability in DAA susceptibility at this level. For example, patient infected with gt1l and gt4r treated with recommended first-line HCV treatment regimens (sofosbuvir + simeprevir, sofosbuvir + daclatasvir, sofosbuvir + ledipasvir, sofosbuvir + velpatasvir, ombitasvir + paritaprevir + ritonavir and grazoprevir + elbasvir) have shown frequent treatment failure, even though these combinations are effective against gt1a, gt1b and gt4a. Among these, sofosbuvir + ledipasvir was the most commonly used regimen, with the cure rate for this subtype in a clinical trial just 56%, compared to 93% for 4k, 90% for 4q, 100% for 4v and 87% for other subtypes]. Similarly, patients infected with subtype 1l appear to be resistant to treatment with sofosbuvir + ledipasvir.
NS5A sequences of the 1l and 4r resistant strains typically encode a particular motif (MRM) at positions 28, 30 and 31 that may be linked to the resistant phenotype. An additional Y93H mutation was also reported in some 4r-infected patients where treatment failed. Subtypes 3b and 3g naturally harbour double NS5A RASs A30K + L31M as the dominant (wild type) sequence. This specific double RASs combination has been shown to confer high level drug resistance when introduced into the 3a subtype raising the possibility that subtypes 3b and 3g are inherently resistant to the approved NS5A inhibitors; this observation needs to be empirically tested. For genotype 6, the most frequently detected subtype, 6a, has been well characterised for treatment response. However, much less is known about the susceptibility of other genotype 6 subtypes. The NS5A sequences of subtypes 6u and 6v harbours amino acid residues reported as RASs in other subtypes but how these specific genotype 6 subtypes respond to DAAs in vitro, or in vivo is unknown.
In the current study we have evaluated the susceptibility of several HCV unusual subtypes in genotypes 1, 3, 4 and 6 against a wide range of currently used NS5A inhibitors. The selected subtypes are not commonly found in North America or Europe where the majority of DAA clinical trials have taken place and which have been referred to as "unusual" subtypes in recent literature and in this manuscript. However, limited data available from countries such as Cameroon (subtype 1l), Rwanda (subtype 4r) [, India (subtype 3g) and Southeast Asia (subtypes 3b, 6u, 6v) suggests that these subtypes are endemic in these parts of the world and collectively pose potential problems with treatment and ultimate eradication of HCV. Documenting their susceptibility to currently used NS5A inhibitors is important for future treatment selection.
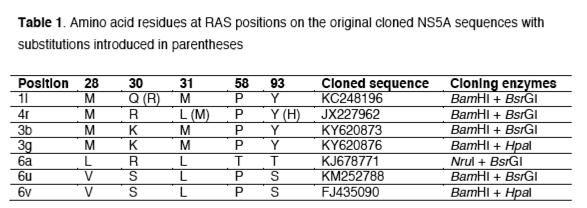
Results
Efficacy of NS5A inhibitors against the tested subtypes
NS5A original sequences of the targeted subtypes were cloned into the SGR-JFH1 backbone for drug testing assays. The tested NS5A inhibitors showed different levels of efficacy against the evaluated subtypes (Figure 1; Table S1). Daclatasvir and ledipasvir showed similar distributions of EC50 values against the tested subtypes: from low (<0.01 nM) against 4r, to moderate (1.8-1.9 nM) against 1l, and high EC50 values (239-14,000 nM) against 3b, 3g, 6u and 6v. Compared to ledipasvir, elbasvir and velpatasvir showed improved efficacy against 1l, 4r, 6u and 6v (EC50 of 0.0001-0.5 nM) but not 3b and 3g (EC50 of 239-2,785 nM). Overall this data suggests that subtypes 3b and 3g may be resistant to these four NS5A inhibitors, whilst 1l, 4r, 6u and 6v may be sensitive to elbasvir and velpatasvir but not to daclatasvir and ledipasvir. Only pibrentasvir had high activity against all tested subtypes.
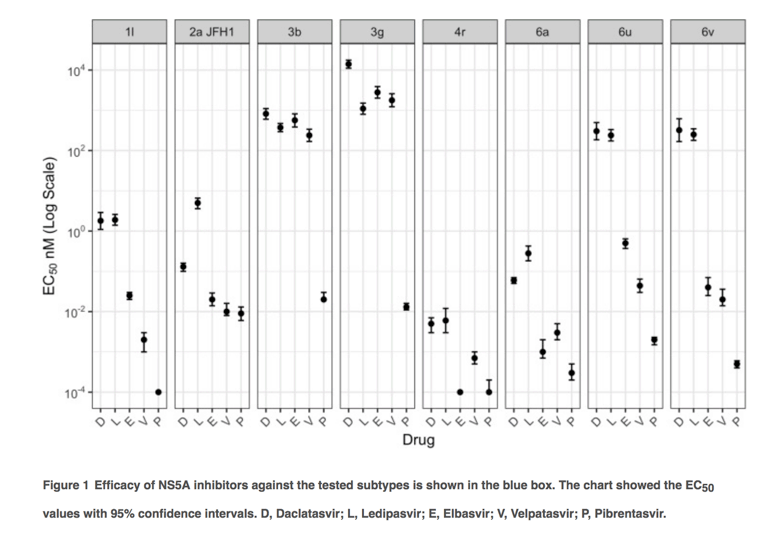
Different subtypes showed differences of up to 7 orders of magnitude in sensitivity to different inhibitors, comparing EC50s of elbasvir of 2,785 nM for the most resistant subtype (3g) with 0.0001 nM for the most sensitive subtype (4r). Daclatasvir, velpatasvir and ledipasvir showed 5-6 log range differences in EC50s. Pibrentasvir showed the smallest range in EC50 values (2 log) with all EC50 values < 0.1 nM for all subtypes testing, and therefore confirming its pan-genotypic efficacy.
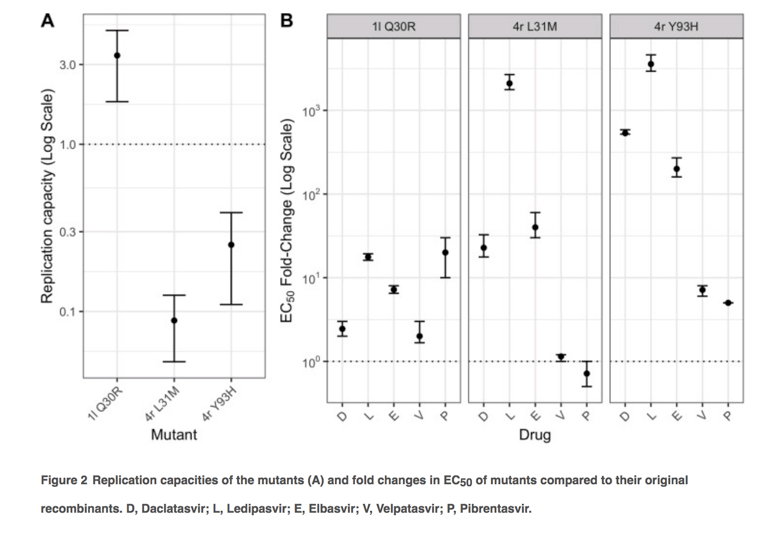
Replication capacities and response of 1l and 4r with reported RASs with NS5A inhibitors
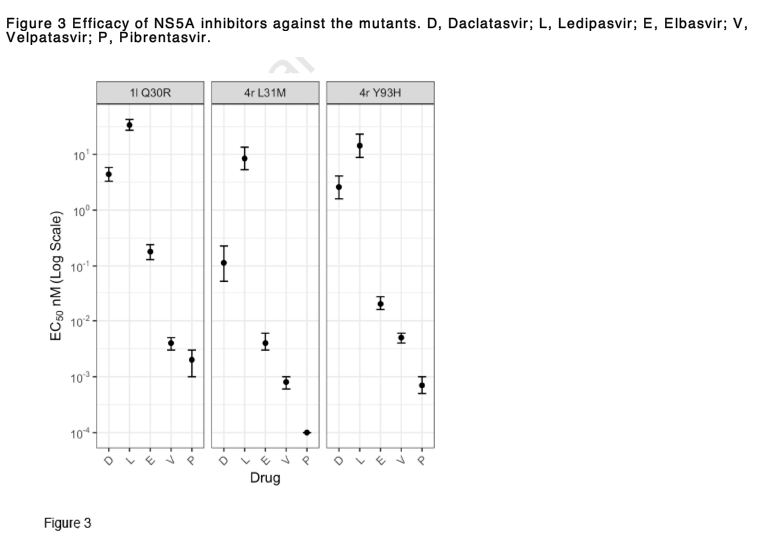
To investigate effects of RASs on replication fitness and DAA susceptibility, we introduced a number of amino acid substitutions in to the 1l and 4r replicons that reproduced those associated with treatment failure (Figure 2). In the 1l replicon, the Q30R substitution actually increased fitness, while the Y93H and particularly L31M substitutions resulted in significant fitness impairment of 4r (Figure 2A). However, the fitness did not correlate with levels of resistance conferred by amino acid substitutions which were classified as low resistance (2-10-fold change), intermediate resistance (11-100-fold change) and high resistance (>100-fold change) [32]. On the basis of this classification, the Q30R substitution in 1l conferred low resistance to daclatasvir, elbasvir and velpatasvir, and intermediate resistance to pibrentasvir and ledipasvir (Figure 2B). Meanwhile, compared to the 4r original recombinant, the mutant with L31M showed variable levels of resistance with different DAAs, ranging from none to pibrentasvir and velpatasvir, intermediate resistance to daclatasvir and elbasvir, and high resistance to ledipasvir. Compared to the 4r original recombinant, the 4r with Y93H substitution showed low resistance to pibrentasvir and velpatasvir, and high level resistance to the other tested inhibitors. Despite the decreased susceptibility to NS5A inhibitors compared to the original recombinants, 1l Q30R and 4r L31M and 4r Y93H were likely sensitive to elbasvir (EC50 ≤ 0.5 nM), velpatasvir and pibrentasvir (EC50 < 0.01 nM) (Figure 3). In contrast, these mutants were partially resistant to ledipasvir (EC50 of 8.4-33.6 nM).
Pibrentasvir is the only drug that was active on all the unusual subtypes-this is invariably given in combination with the NS3 inhibitor glecaprevir. We therefore evaluated in silico, for the presence of RAS in NS3 that may be resistant to glecaprevir and that if present may lead to resistance to this drug combination. We downloaded 15, 98, 4, 17, 6 and 6 NS3 sequences from 1l, 3b, 3g, 4r, 6u, 6v, respectively, from Genbank, for analysis. As the resistance profiles of these subtypes have not been well studied, we defined a RAS as a polymorphism known to incur resistance (as reported in http://hcv-glue.cvr.gla.ac.uk/#/project/rap) in the most closely related common subtype (e.g. the resistance profile of 3a was used to analyse 3b and 3g NS3 sequences) and analysed amino acid residues at these positions. We found only the A166S RAS in 3/98 and 4/4 NS3 sequences from 3b and 3g, respectively.
Discussion
This study reports the efficacy of currently approved NS5A inhibitors, including second generation pan genotypic inhibitors such as pibrentasvir and velpatasvir against under studied, unusual, HCV subtypes 1l, 3b, 3g, 4r, 6u and 6v.
Although studies have overall reported high SVR rates with DAAs in patients with gt4 infection, subtype 4r has been reported to be less responsive to DAAs than other genotype 4 subtypes, resulting in treatment failure in up to 44% in patients treated with sofosbuvir + ledipasvir in a recent trial. Similarly, 1l-infected patients have been shown to be less responsive to sofosbuvir + ledipasvir. HCV strains from these patients commonly harboured RASs found at positions 28, 30, 31 and 93 of the NS5A sequences at both baseline and treatment failure. The NS5A motif M28R30M31Y93 in subtypes 1l and 4r and M28R30L31H93 in subtype 4r were associated with treatment failure. We recreated these motifs as 1l Q30R, 4r L31M and 4r Y93H variants. The ledipasvir EC50 values of these mutants were 33.6, 8.4 and 14.3 nM, respectively, which may explain failure in the treated patients. This is comparable with the ledipasvir EC50 of 9.5 nM previously reported from 4r with M28R30M31. Although all 4r variants in this study were sensitive to velpatasvir, regimens with triple DAA combinations should be considered for these subtypes as treatment failure has occurred with many treatment regimens, including sofosbuvir + velpatasvir + ribavirin.
Our previous study highlighted the need for more study into the efficacy of DAA therapy in less common genotype 3 subtypes. In this study, subtypes 3b and 3g had high EC50 values (239-14,000 nM) of daclatasvir, elbasvir, ledipasvir and velpatasvir, reinforcing their potential of resistance to these DAAs. These findings support the recent recommendation from the European Association for the Study of the Liver for additional use of NS3 inhibitors for treatment of genotype 3-infected patients (e.g. sofosbuvir + velpatasvir + voxilaprevir) or use of pibrentasvir with glecaprevir, an NS3 inhibitor. However, voxilaprevir is only required for patients with compensated cirrhosis and the RAS Y93H. This study together with our previous study suggests that voxilaprevir should also be considered for treatment of patients with the RAS combination A30K + L31M, which is naturally present in the 3b, 3g NS5A sequences and has been shown to provide high resistance (> 10,000-fold increase in EC50) to velpatasvir compared to the wild type 3a.
Genotype 6, the most diverse genotype with 24 currently classified subtypes, is most prevalent in Southeast Asia. Clinical trials and real-world treatment data on limited numbers of genotype 6 patients showed high SVR12 rates. However, health budget restrictions in Southeast Asian countries have limited the use of the recently recommended treatment regimens of sofosbuvir + ledipasvir, sofosbuvir + velpatasvir or glecaprevir + pibrentasvir, and generic and less costly versions of sofosbuvir + ledipasvir are more widely used. The Drugs for Neglected Disease initiative (DNDi) has developed a novel combination therapy for use in middle/lower income countries with ravidasvir, an NS5A inhibitor with a similar in vitro profile to daclatasvir, as a component. Several ongoing clinical trials in Vietnam where genotype 6 infections are prevalent use sofosbuvir + ravidasvir. It will be important to identify response rates to individual genotype 6 subtypes in patients treated with these generic drugs from both clinical trials and real world treatment data. Subtypes 6u and 6v were poorly inhibited in vitro by daclatasvir and ledipasvir in this study and these and potentially other gt6 subtypes may be systematically more resistant to DAA therapy.
We show that only pibrentasvir is efficacious against all tested unusual subtypes. However, this is usually given in combination with the NS3 inhibitor glecaprevir. There is currently limited data on NS3 sequences available for these unusual subtypes and therefore the NS3 inhibitor resistance profile is not well characterised. In an in silico analysis, we show that all 3g and some 3b NS3 sequences harbour the A166S RAS. The presence of this NS3 RAS at baseline could impact on the efficacy of glecaprevir and thus treatment with the "pan-genotypic" therapy with glecaprevir/pibrentasvir.
Overall, this work demonstrates that these understudied subtypes have reduced susceptibilities to commonly used NS5A inhibitors such as ledipasvir and velpatasvir. Clinical studies and careful clinical evaluation is needed to confirm in vivo sensitivity to NS5A inhibitors are urgently needed to ensure effective treatment strategies for less common subtypes where in vitro and/or clinical resistance has been demonstrated. Until then, our data supports the (EASL) in full recommendations that DAA combinations that include the second generation NS5A inhibitors such as glecaprevir + pibrentasvir and sofosbuvir + velpatasvir should be used in the treatment of these subtypes.
| |
| |
| |
|
|
|