| |
Antiretroviral Therapy-Induced Bone Loss Is Durably Suppressed by a Single Dose of Zoledronic Acid in Treatment-Naive Persons with Human Immunodeficiency Virus Infection: A Phase IIB Trial
|
| |
| |
Download the PDF here
In this study, we extend our findings reported previously through the first 48 weeks following ART initiation [19]. We demonstrate that ART-associated bone resorption and BMD loss extend beyond the first 2 years of therapy, and that a single dose of ZOL given at the time of therapy initiation durably blunts these effects.
Clinical Infectious Diseases October 2020
Ighovwerha Ofotokun,1,2,a Lauren F. Collins,1,2 Kehmia Titanji,3 Antonina Foster,1 Caitlin A. Moran,1,2 Anandi N. Sheth,1,2 Cecile D. Lahiri,1,2
Jeffrey L. Lennox,1,2 Laura Ward,4 Kirk A. Easley,4 and M. Neale Weitzmann3,5,a
1Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA, 2Grady Health System, Atlanta, Georgia, USA, 3Division of Endocrinology
and Metabolism and Lipids, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA, 4Department of Biostatistics and Bioinformatics, Rollins School of Public Health,
Emory University, Atlanta, Georgia, USA, and 5Atlanta Department of Veterans Affairs Medical Center, Decatur, Georgia, USA
Abstract
Background
Human immunodeficiency virus (HIV) infection and antiretroviral therapy (ART) are associated with bone loss leading to increased fracture rate among persons with HIV (PWH). We previously showed long-acting antiresorptive zoledronic acid (ZOL) prevented ART-induced bone loss through 48 weeks of therapy and here investigate whether protection persisted.
Methods
We randomized 63 nonosteoporotic, treatment-naive adult PWH initiating ART to ZOL (5 mg) versus placebo in a double-blinded, placebo-controlled, phase IIb trial. Here we analyzed the long-term outcome data (144 weeks). Plasma bone turnover markers and bone mineral density (BMD) were quantified at weeks 0, 12, 24, 48, 96, and 144. Primary outcome was change in bone resorption marker C-terminal telopeptide of collagen (CTx). Repeated-measures analyses using mixed linear models were used to estimate and compare study endpoints.
Results
At 96 weeks, mean CTx was 62% lower with ZOL relative to placebo (n = 46; CTx = 0.123 vs 0.324 ng/mL; P < .001); at 144 weeks a 25% difference between arms was not statistically significant. At 48 weeks, lumbar spine BMD with ZOL was 11% higher than placebo (n = 60; P < .001) and remained 9-11% higher at 96 (n = 46) and 144 (n = 41; P < .001) weeks. 144 weeks after ZOL infusion, BMD did not change at the lumbar spine (P = .22) but declined at the hip (P = .04) and femoral neck (P = .02).
Conclusions
A single dose of ZOL administered at ART initiation blunts bone resorption and BMD loss at key fracture-prone anatomical sites in treatment-naive PWH for 3 years. A multicenter randomized phase III clinical trial validating these results in a larger population is needed.
DISCUSSION
In this study, we extend our findings reported previously through the first 48 weeks following ART initiation [19]. We demonstrate that ART-associated bone resorption and BMD loss extend beyond the first 2 years of therapy, and that a single dose of ZOL given at the time of therapy initiation durably blunts these effects.
Prior studies have shown the vast majority of ART-associated bone loss occurs 1-2 years after therapy initiation, with subsequent stabilization in BMD thereafter [20]. In this report, ART initiation led to a surge in bone resorption in patients randomized to the placebo arm, peaking at 24 weeks and persisting through 96 weeks. ZOL ameliorated this increase resorption, resulting in a 62% reduction in mean bone resorption at 96 weeks, and 9-11% higher lumbar spine BMD at 96-144 weeks, relative to placebo. Current guidance for the management of bone disease in HIV provides basic recommendations for all PWH and those specifically diagnosed with osteoporosis [10]. For PWH initiating ART, it is recommended to avoid certain antiretrovirals known to be detrimental to bone (ie, TDF and protease inhibitors) [10]. Our findings suggest that additional strategies to minimize the risk of ART-induced skeletal deterioration may be warranted, such as prophylactic antiresorptive therapy, and that the benefits of such intervention may extend beyond the first 2 years following ART initiation. Such preemptive therapy may be of particular benefit to PWH with multiple risk factors for fragility bone disease or with low BMD at the onset of ART.
The rate of loss in BMD experienced by PWH initiating ART is similar to that seen during the rapid phase of bone loss in postmenopausal osteoporosis [21]. However, the pathogenesis of bone loss in these groups differs starkly and, furthermore, HIV-specific demographic and clinical characteristics require special consideration. First, bone loss and fragility fracture in the setting of HIV infection occur at a much younger age, with data [6] suggesting at least a decade earlier than HIV-seronegative individuals [3, 6]. Second, the skeletal health of PWH is compromised at baseline (pre-ART) due to a variety of intrinsic and extrinsic factors [9, 22]. Intrinsically, effects of HIV on inflammation, the adaptive immune dysregulation, and disruption in the integrity of the immunoskeletal interphase have been well described [23-26]. Direct viral effects on bone were recently reported by Raynaud-Messina et al [27]. Extrinsically, traditional risk factors for low BMD are highly prevalent in PWH, further compounding the risk of fragility bone disease in this population [28]. Finally, initiation of ART paradoxically worsens bone health [14, 29]. These considerations underscore the pressing need to develop HIV-specific preventive and therapeutic strategies for alleviating bone disease in this high-risk population. Further, an epidemic of other metabolic comorbidities and aging add urgency to this clinical priority for PWH [8, 30].
The role of calcium/vitamin D supplementation in improving skeletal health in PWH remains unclear and may depend on baseline 25-hydroxyvitamin D levels, ART status, and dosing of supplementation [23-25]. Our data suggest that the use of bisphosphonates to reduce ART-induced bone loss in PWH is promising. While we observed a significant decrease in lumbar spine BMD of PWH initiating ART in the placebo arm at 144 weeks, no change was observed in lumbar spine BMD in the ZOL arm; however, significant increases in hip and femoral neck BMD were found in ZOL-treated PWH at this time point. Zoledronic acid has also been studied in ART-treated PWH with low BMD and was shown to be superior to TDF-switching at increasing BMD [26]. A study of ART-treated men with HIV with low BMD at baseline who received 2 annual doses of ZOL (4 mg) demonstrated favorable effects on bone turnover and BMD that persisted over nearly 2 years [31].
Although the sample size was relatively small, ZOL did not suppress bone formation in our study population as has been previously reported in other studies [28]. This is important because prolonged use of bisphosphonates may cause low bone turnover that has been associated with poorly remodeled bone susceptible to microcracks [32]. Further, ZOL at a single dose was safe and well tolerated, and we observed comparable virologic suppression and magnitude of CD4+ T-cell reconstitution between treatment arms.
Our phase IIb clinical trial was a proof-of-concept study conducted at a single site and therefore has several limitations. Generalizability of our findings is limited by the small sample size and homogeneous study population (eg, predominantly African-American men). The 144-week study duration could not evaluate the impact of our intervention on long-term bone outcomes such as fracture.
In conclusion, a single infusion of ZOL at the time of ART initiation blunted ART-induced bone resorption and prevented bone loss in nonosteoporotic PWH. Importantly, preserved BMD was observed at key fracture-prone anatomical sites through week 144, during which time ZOL was well tolerated. These data provide a robust framework for the design and execution of larger confirmatory phase 3, multicenter, randomized clinical trials investigating the efficacy of antiresorptive therapy in mitigating ART-induced bone loss in PWH
Serious Adverse Effects, Adverse Effects, and Laboratory Toxicities
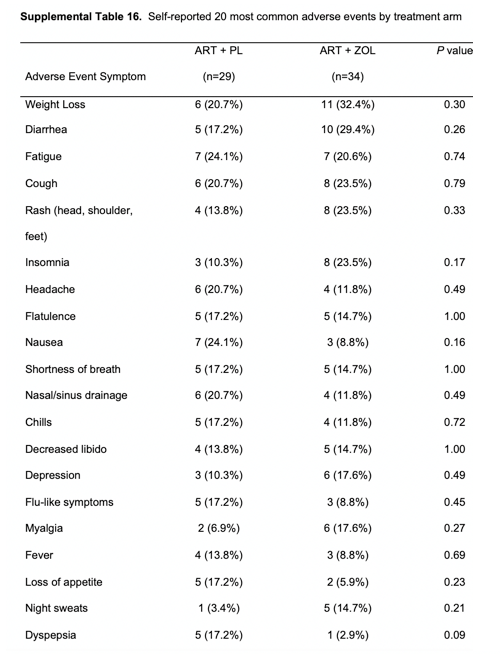
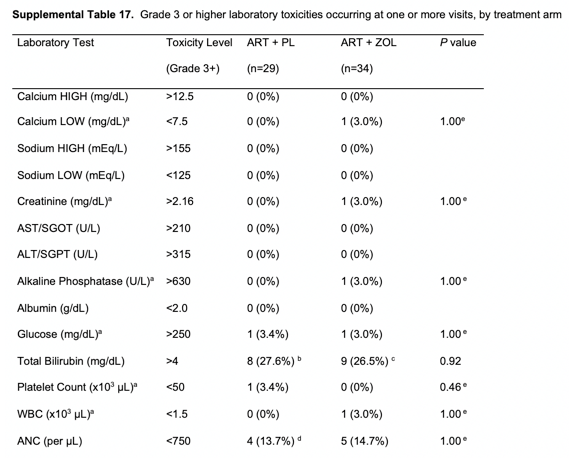
Over the 144 weeks of follow-up, no serious adverse effects (SAEs) were reported to be possibly or definitively related to ZOL treatment. Serious adverse effects were similar between the ZOL and placebo arms. Supplementary Table 16 summarizes patient-reported adverse effects by treatment arm. Supplementary Table 17 summarizes laboratory toxicities. There were no statistically significant differences between treatment arms for the incidence of any grade 3 or higher laboratory toxicities during 144 weeks of follow-up.
Bone Formation Was Not Adversely Impacted by Zoledronic Acid
Osteocalcin in the 2 treatment groups changed in similar ways (similar temporal patterns over time) during follow-up (P = .35, test for interaction between time on study and treatment group). A compensatory increase in osteocalcin was observed in the placebo arm but not in the ZOL arm (P = .007) when testing the time-averaged differences between the 2 treatment groups. Mean difference in osteocalcin between the arms pooled over the 144-week follow-up period was -4.2 ng/mL (95% CI, -10.2 to -1.9 ng/mL) (Figure 2C). While mean differences in osteocalcin at 12, 24, and 48 weeks differed significantly [19], they did not significantly differ at 96 or 144 weeks (P = .08 and P = .18, respectively).
Respective mean differences at 96 and 144 weeks were -4.7 ng/mL (95% CI, -10.0 to 0.6 ng/mL) and -4.1 ng/mL (95% CI, -10.1 to 1.9 ng/mL). In the placebo arm, the osteocalcin mean percentage increases from baseline to 96 and 144 weeks were 95% (95% CI, 21-169%) and 55% (95% CI, 18-92%), respectively. The osteocalcin mean percentage increases from baseline to 96 and 144 weeks did not change in the ZOL arm: 113% (95% CI, -44% to 271%) and 91% (95% CI, -29% to 211%), respectively (Figure 2D; Supplementary Tables 4 and 5).
Zoledronic Acid Prevented Antiretroviral Therapy-Induced Bone Mineral Density Loss
Lumbar spine BMD in the treatment arms changed in significantly different ways (ie, different temporal patterns over time) during the 144 weeks of follow-up (P < .001, test for interaction between time on study and treatment arm). Mean lumbar spine BMD was similar in both treatment arms at randomization (P = .08) but became significantly higher in the ZOL arm at 12, 24, and 48 weeks [19], and this effect persisted through 96 weeks (1.299 vs 1.189 g/cm2; P < .001) and 144 weeks (1.306 vs 1.177 g/cm2; P < .001) (Figure 3A). Zoledronic acid significantly increased lumbar spine BMD at 12, 24, and 48 weeks [19] and, relative to placebo, led to a mean difference in lumbar spine BMD at 96 and 144 weeks of 0.111 g/cm2 (9% increase) and 0.129 g/cm2 (11% increase), respectively. Bone mineral density at the lumbar spine did not change from baseline to 144 weeks in the ZOL arm (mean percentage increase of 1.0%; 95% CI, -0.61% to 2.61%; P = .22) but decreased by -4.3% (95% CI, -6.48% to -2.15%; P < .001) in the placebo arm (Figure 3B; Supplementary Tables 6-9). In the ZOL arm, the hip and the femoral neck BMD was preserved up to week 48; however, a small but significant decline from baseline by 144 weeks was observed at these sites—for the hip BMD: mean decline, 0.016 g/cm2; (95% CI, 0.001-0.031; P = .04; for the femoral neck BMD: mean decline, 0.021 g/cm2; 95% CI, 0.003-0.041; P = .02 (Supplementary Figure 1; Supplementary Tables 10-14). At baseline, the number of particpants with osteopenia (T-score between -1.0 and -2.5) in any area in the placebo arm was 10 (34.5%) and was 7 (21.9%) in the ZOL arm. No participant was osteoporotic (T-score ≤ -2.5) at baseline. The 29 participants in the ZOL arm who completed the week-144 visit maintained their baseline BMD status (number with osteopenia, 6; 21%). By contrast, of the 20 participants who completed the week-144 visit in the placebo arm, 8 (40%) were osteopenic (T-score between -1.0 and -2.5) and 1 participant developed osteoporosis.
|
|
| |
| |
|
|
|