 |
 |
 |
| |
Dolutegravir With TAF or TDF Could
Increase Adverse Pregnancy Outcomes
|
| |
| |
AIDS 2020: 23rd International AIDS Conference Virtual, July 6-10, 2020
Mark Mascolini
Regimens containing the integrase inhibitor dolutegravir (DTG) plus tenofovir alafenamide (TAF) or tenofovir disoproxil fumarate (TDF) could boost long-term risk of adverse pregnancy outcomes for mothers and infants, according to analysis of the South African ADVANCE trial plus a systematic review of adverse outcomes with obesity in pregnant women [1]. In the ADVANCE trial obesity developed in women taking DTG/TAF/emtricitabine (FTC) almost twice as often as in women taking DTG/TDF/FTC.
Research links DTG and other integrase inhibitor to significant weight gain, especially when combined with TAF and especially in women and blacks. Pregnant women who become obese run an increased risk of adverse pregnancy outcomes, but short-term results of DTG/TAF/FTC studies in pregnant women did not disclose a significantly higher risk of adverse birth outcomes. ADVANCE trial researchers analyzed long-term data from that trial to determine long-term consequences of DTG/TAF/FTC and DTG/TDF/FTC versus a non-DTG regimen on maternal weight gain and adverse pregnancy outcomes.
ADVANCE randomized 1053 antiretroviral-naive nonpregnant South African adults and adolescents to DTG/TAF/FTC, DTG/TDF/FTC, or efavirenz (EFV)/TDF/FTC [2]. Almost all study participants (99%) were black, and 59% were women. After 48 weeks proportions with a viral load below 50 copies were 84% with DTG/TAF/FTC, 85% with DTG/TDF/FTC, and 79% with EFV/TDF/FTC, a result establishing the virologic noninferiority of the DTG regimens to the EFV combination.
Through 144 weeks of follow-up in ADVANCE women, average weight rose more with DTG/TAF/FTC (12.3 kg) than with DTG/TDF/FTC (7.4 kg) or EFV/TDF/FTC (5.5 kg). At week 96 proportions of women with treatment-emergent obesity were 14% with DTG/TAF/FTC, 8% with DTG/TDF/FTC, and 2% with EFV/TDF/FTC.
Next the researchers conducted a systematic review of adverse birth outcomes in obese women (at or above 30 kg/m2) versus normal-weight women (18.5 to 24.9 kg/m2). They searched online databases for cohort studies that assessed the impact of maternal obesity on adverse pregnancy outcomes then compared the risk of adverse outcomes in obese versus normal-weight women. The investigators selected 25 studies for analysis.
Taking gestational diabetes as an example of an adverse pregnancy outcome, the researchers noted that the background risk of gestational diabetes by systematic review is 1.6%. In other words, for 1000 pregnant women with normal weight, gestational diabetes would be expected to develop in 16 women. Obesity inflates the risk of gestational diabetes 6.9%, which equals 1.6 x 4.31 (the relative risk by systematic review). If 1000 pregnant women with normal body weight took DTG/TAF/FTC for 96 weeks, ADVANCE results indicate that 14% (140 women) would become obese after 96 weeks and 86% (860 women) would not become obese after 96 weeks. Applying the 6.9% increase in gestational diabetes risk with obesity to 140 women would yield 9.6 cases. Applying the 1.6% background risk of gestational diabetes to 860 nonobese women would yield 13.8 cases. Adding the 9.6 cases in obese women to the 13.8 cases in nonobese women would yield 23.4 cases of gestational diabetes per 1000 women.
Systematic review set the following relative risks of adverse pregnancy outcomes for obese versus normal-weight pregnant women: preterm delivery, 1.33; gestational hypertension, 3.68; gestational diabetes, 4.31; preeclampsia, 4.06; postpartum hemorrhage, 1.23; and Caesarean section, 1.64. For 1000 pregnancies in the ADVANCE trial population, the predicted increased risk in 96-week adverse pregnancy outcomes with each regimen versus the baseline rate would be:
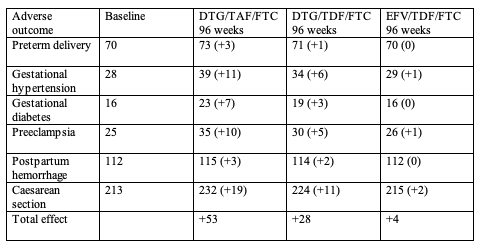
By systematic review the researchers figured the following relative risks of adverse infant outcomes for infants of obese versus normal-weight mothers: large for gestational age, 2.04; macrosomia, 2.48; small for gestational age, 0.84; neonatal death, 1.57; stillbirth, 1.39; and neural tube defect, 2.53. For 1000 pregnancies in the ADVANCE trial population, the predicted 96-week adverse infant outcomes with each regimen versus the baseline rate would be;
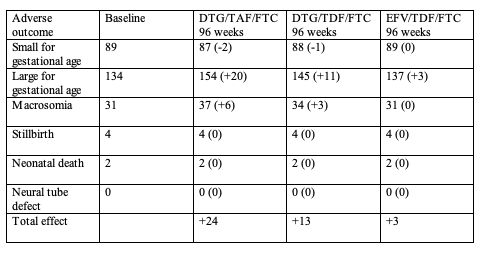
Summarizing the main findings, the ADVANCE team stressed that 14% of women with normal pretreatment weight in that trial became obese after 96 weeks of DTG/TAF/FTC versus 8% taking DTG/TDF/FTC and 2% taking EFV/TDF/FTC. Systematic review confirmed the increased risk of adverse maternal and infant outcomes when women are obese during pregnancy. Applying increased adverse pregnancy outcomes derived from systematic review to 1000 women and infants in the ADVANCE population, the researchers calculated that long-term treatment with DTG/TAF/FTC would add 77 adverse outcomes per 1000 births with DTG/TAF/FTC and 41 adverse outcomes per 1000 births with DTG/TDF/FTC. And those rates could grow if women take these dolutegravir regimens longer than 96 weeks.
References
1. Asif S, Baxevanidi E, Hill A, et al. The predicted risk of adverse pregnancy outcomes from treatment-induced obesity in the ADVANCE trial. AIDS 2020: 23rd International AIDS Conference Virtual. July 6-10, 2020. Abstract OABLB0103.
2. Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381:803-815. doi: 10.1056/NEJMoa1902824.
|
| |
|
 |
 |
|
|