 |
 |
 |
| |
Risk Factors and Metabolic Implications of Integrase Inhibitor Associated Weight Gain
|
| |
| |
Integrase Inhibitors May Triple Diabetes Risk in First 18 Months of Therapy
IDWeek 2020, October 22-25, 2020
Mark Mascolini
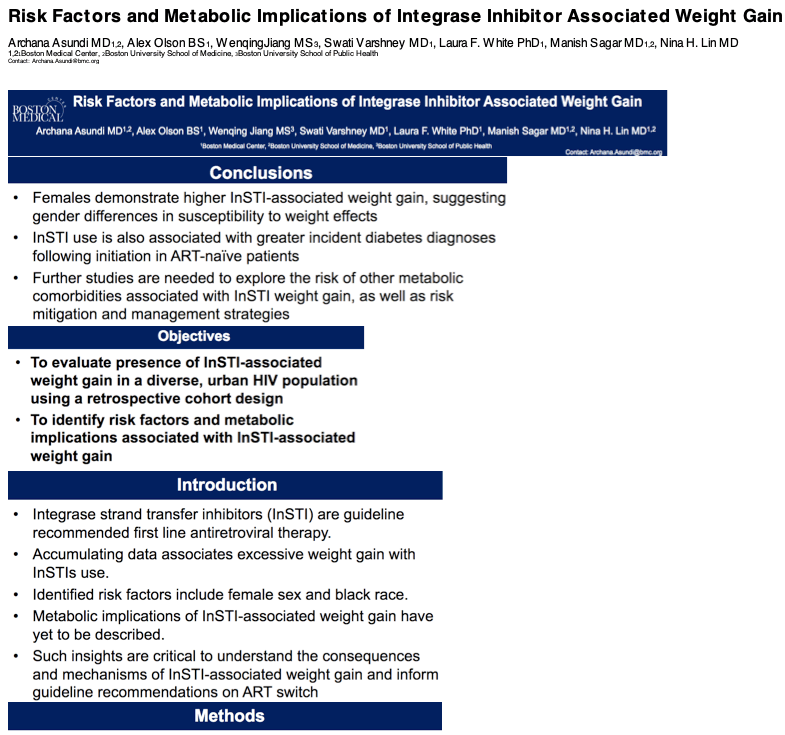
Compared with first-line antiretroviral regimens not including an integrase inhibitor (InSTI), those that did tripled the risk of a new diabetes diagnosis in a 612-person retrospective Boston study [1]. Both white and nonwhite women starting antiretroviral therapy with InSTIs gained significantly more weight in the first 24 months of therapy than women starting non-InSTI combinations.
Previous research found that women and blacks run a higher risk of weight gain with InSTIs. But InSTI impacts on other metabolic outcomes, including new-onset (incident) diabetes, remain to be established. Study of 19,462 HIV-positive people in France starting first-line therapy with or without an InSTI found no link between InSTIs and new-onset diabetes [2]. But a recent analysis of the FDA Adverse Event Reporting System (FAERS) tied the InSTIs bictegravir and dolutegravir to hyperglycemia or new-onset diabetes [3].
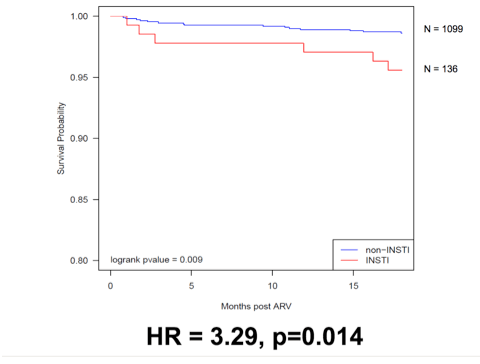
A new retrospective cohort study by researchers at Boston Medical Center and Boston University compared antiretroviral-naive adults starting an InSTI with those starting a non-InSTI regimen in fiscal years 2007-2017. Participants had to stay on their initial regimen for at least 18 months. No one had diabetes before they started antiretroviral therapy. The Boston team used a linear mixed-effects model to compare the two groups for weight change through the first 24 months of therapy. They used Kaplan-Meier-determined progression-free survival and a Cox proportional hazards model to compare the groups for new-onset diabetes in the first 18 months of therapy.
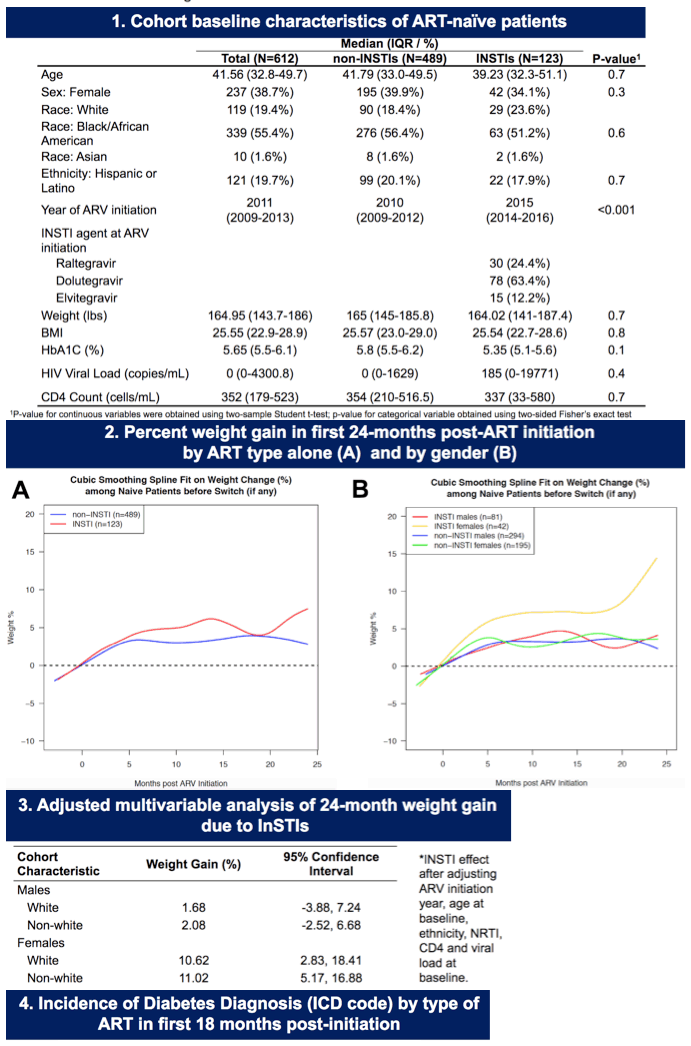
The analysis involved 123 people starting an InSTI regimen and 489 starting a non-InSTI combination. The two groups differed little in proportions of women (39% overall), blacks (55%), Hispanics (20%), weight (median 165 lbs), body mass index (median 25.5 kg/m2), HbA1c, a blood sugar measure (5.65%), or CD4 count (median 352).
Multivariable analysis determined that women taking InSTIs gained significantly more weight in the first 24 months of therapy than women taking a non-InSTI combination: +10.62% (95% confidence interval [CI] 2.83 to 18.41) for white women and +11.02% (95% CI 5.17 to 16.88) for nonwhite women. This analysis adjusted for year treatment began, ethnicity, nucleos(t)ides in regimen, and pretreatment age, CD4 count, and viral load. Men starting an InSTI combination did not gain significantly more weight than men starting other regimens.
After the first 18 months of therapy, InSTI regimens were associated with a 3-fold higher risk of new-onset diabetes determined by ICD codes (adjusted hazard ratio 3.29, P = 0.014).
The Boston team suggested that the greater InSTI-related weight gain in women reflects gender differences in susceptibility to the weight effects of these antiretrovirals. They called for further study to uncover the mechanisms behind weight and metabolic changes with InSTIs and to inform risk mitigation and management.
References
1. Asundi A, Olson A, Jiang W, et al. Risk factors and metabolic implications of integrase inhibitor associated weight gain. IDWeek 2020, October 22-25, 2020. Abstract 946.
2. Ursenbach A, Max V, Maurel M, et al; Dat'AIDS Study Group. Incidence of diabetes in HIV-infected patients treated with first-line integrase strand transfer inhibitors: a French multicentre retrospective study. J Antimicrob Chemother. 2020 Aug 13:dkaa330. doi: 10.1093/jac/dkaa330.
3. Murray MM, Harpe SE, META-INSTI: metabolic adverse events following integrase strand transfer inhibitor administration in spontaneous adverse event reports. IDWeek 2020, October 22-25, 2020. Abstract 106.

|
| |
|
 |
 |
|
|