 |
 |
 |
| |
Weight change associated with switching to bictegravir/emtricitabine/tenofovir alafenamide
in virally suppressed people with HIV
Weight Climbs With Switch to Bictegravir Across Baseline Regimens
|
| |
| |
IDWeek 2020, October 22-25, 2020
Mark Mascolini
Body mass index (BMI) rose significantly after a switch to the integrase inhibitor bictegravir compared with weight trends during treatment with a nonintegrase regimen, a nonnucleoside (NNRTI) regimen, or a regimen containing tenofovir disoproxil fumarate (TDF) [1]. Higher proportions switching to bictegravir had at least a 10% jump in BMI if switching from a non-protease inhibitor (PI) regimen or a TDF regimen.
Bictegravir coformulated with emtricitabine and tenofovir alafenamide (B/F/TAF, Biktarvy) has emerged as a popular 1-pill-once-daily antiretroviral regimen. Although plentiful evidence documents weight gains when switching to other integrase inhibitors, data on bictegravir switches remain relatively scant.
A randomized comparison of switching to bictegravir or dolutegravir found similar small weight gains (1.3 and 1.1 kg) with either regimen at 48 weeks [2]. An 8-trial 5680-person pooled analysis of people starting their first antiretrovirals found similar 96-week weight gains with bictegravir and dolutegravir (4.24 and 4.07 kg), both higher than with elvitegravir/cobicistat (2.72 kg) [3]. Compared with efavirenz, odds of at least a 10% weight gain proved greater with bictegravir and dolutegravir (1.82-fold) than with elvitegravir/cobicistat (1.36-fold) or the NNRTI rilpivirine (1.51-fold).
Researchers at Washington University in St. Louis focused on weight change in virally suppressed people switching to B/F/TAF from February 2018 to February 2020. Everyone had at least two weight measurements before and after the switch. The research team used linear mixed effects models to assess the BMI impact of switching to B/F/TAF overall and according to baseline antiretrovirals.
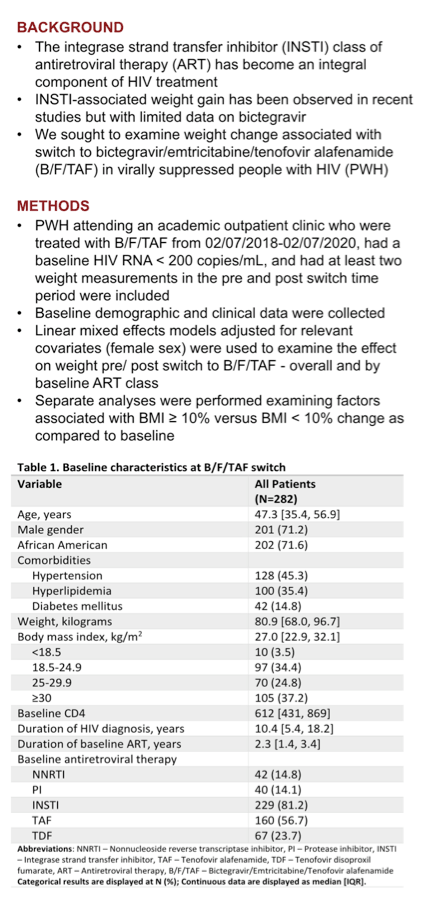
The 282 study participants had a median age of 47.3, 71% were men and 72% African American. At the switch median weight stood at 80.9 kg (178 pounds), 25% were overweight, and 37% were obese. Median time since HIV diagnosis measured 10.4 years, and people took the baseline regimen for a median of 2.3 years. A large majority of participants (81%) took an integrase inhibitor in their baseline regimen (54% elvitegravir, 43% dolutegravir), while 15% took an NNRTI, 14% a PI, 57% TAF, and 24% TDF. Studies consistently show bigger weight gains with TAF than TDF.
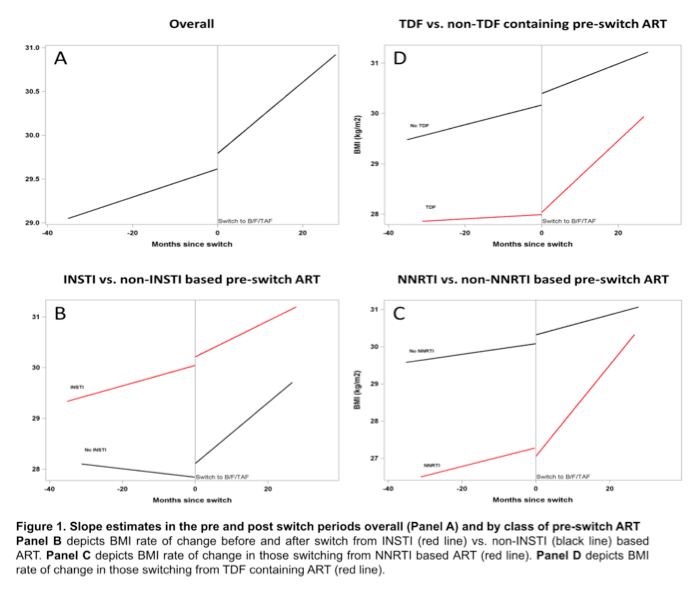
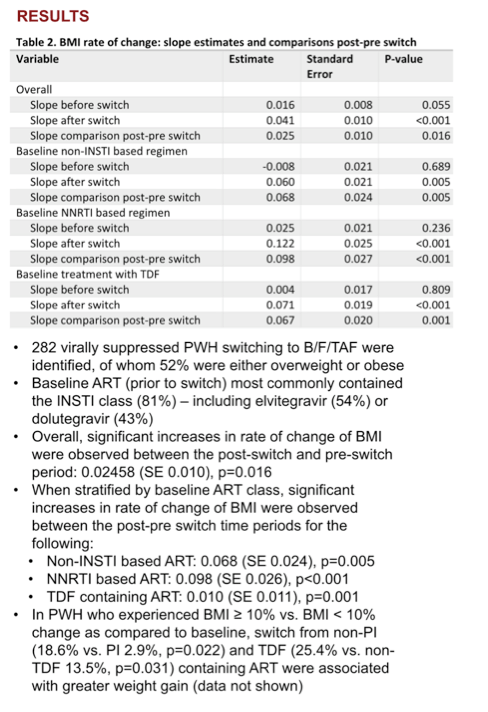
Overall estimated BMI rate of change was slow before the switch to B/F/TAF (0.016, P = 0.055) and fast after the switch (0.041, P < 0.001) for a significant pre/post-switch difference of 0.025 (P = 0.016). Among people taking a baseline regimen not including an integrase inhibitor, the preswitch weight slope was flat (-0.008, P = 0.689), then jumped after the switch to B/F/TAF (0.060, P = 0.005) for a pre/post difference of 0.068 (P = 0.005).
The weight-change slope trended modestly upward in people taking a baseline NNRTI combination (0.025, P = 0.236) then veered sharply upward after the switch to B/F/TAF (0.122, P < 0.001) for a pre/post difference of 0.098 (P < 0.001). Among people taking TDF in their baseline combination, an essentially flat preswitch slope (0.004, P = 0.809) moved steeply upward after the switch (0.071, P < 0.001) for a difference of 0.067 (P = 0.001).
A higher proportion of participants had a 10% or greater BMI gain when switching to B/F/TAF from a non-PI regimen than from a PI (18.6% vs 2.9%, P = 0.022) or from a TDF regimen than from a non-TDF regimen (25.4% vs 13.5%, P = 0.031).
The Washington University group concluded that switching from a virologically effective combination to B/F/TAF leads to significantly steeper upward BMI slopes, particularly in people switching from a nonintegrase regimen containing an NNRTI.
References
1. Vo D, Goss CW, Lian Q, O'Halloran JA. Weight change associated with switching to bictegravir/emtricitabine/tenofovir alafenamide in virally suppressed people with HIV. IDWeek 2020, October 22-25, 2020. Abstract 1047.
2. Sax PE, Rockstroh JK, Luetkemeyer AF, et al. Switching to bictegravir, emtricitabine, and tenofovir alafenamide in virologically suppressed adults with HIV. Clin Infect Dis. 2020 Jul 15:ciaa988. doi: 10.1093/cid/ciaa988.
3. Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. 2020;71:1379-1389. doi: 10.1093/cid/ciz999.
|
| |
|
 |
 |
|
|