 |
 |
 |
| |
Preexisting resistance and week 48 virologic outcomes after switching to B/F/TAF in African American adults with HIV - Frequent Resistance Before B/F/TAF Does Not Dim Week-48 Viral Response
|
| |
| |
IDWeek 2020, October 22-25, 2020
Mark Mascolini
Despite frequent detection of resistance mutations before a switch to the 3-in-1 antiretroviral bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF), nearly everyone assessed after 48 weeks had a viral load below 50 copies [1]. No new resistance mutations emerged in this ongoing study of 495 African Americans.
Anchored on the integrase inhibitor bictegravir, single-tablet B/F/TAF ranks as an attractive option for adults and children with HIV. As a switch-to regimen, B/F/TAF may raise concerns that detectable or archived resistance mutations will emerge with B/F/TAF, especially since so many people have taken failed regimens containing lamivudine (3TC) or emtricitabine (FTC), which can give rise to the M184V/I mutation, or containing tenofovir disoproxil fumarate (TDF), which can allow emergence of K65 mutations threatening TAF.
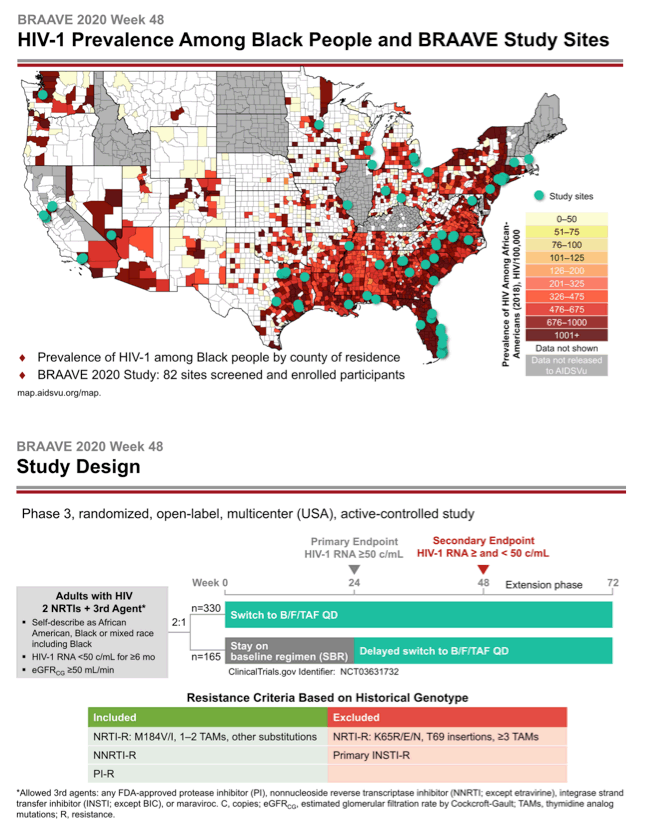
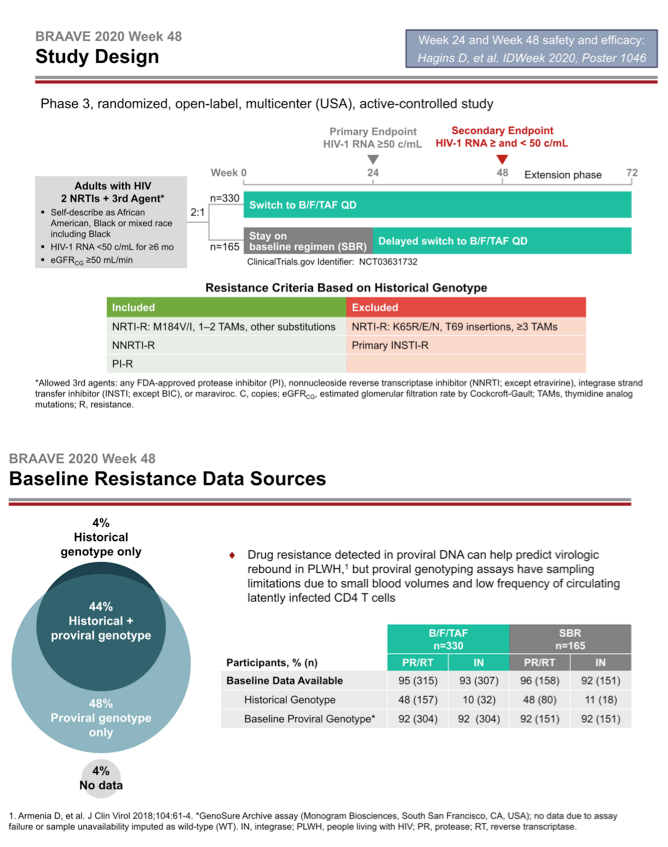
BRAAVE 2020 is assessing response to B/F/TAF in US blacks switching from an HIV-suppressing regimen [2]. Among US racial and ethnic groups, blacks have the highest HIV rates in the country and have not been well represented in clinical trials. This trial allowed enrollment of people with prior antiretroviral failure (except integrase inhibitor failure) and with documented resistance to nonnucleosides (NNRTIs), protease inhibitors (PIs), and nucleosides or nucleotides (NRTIs) (except those with the TDF-linked K65R/E/N mutations, 3 or more thymidine analog mutations, T69-insertion mutations, or primary integrase inhibitor mutations). Researchers checked trial candidates for resistance with historical genotypes (resistance mutations recorded in the past) and/or proviral DNA genotyping (which may detect hidden mutations).
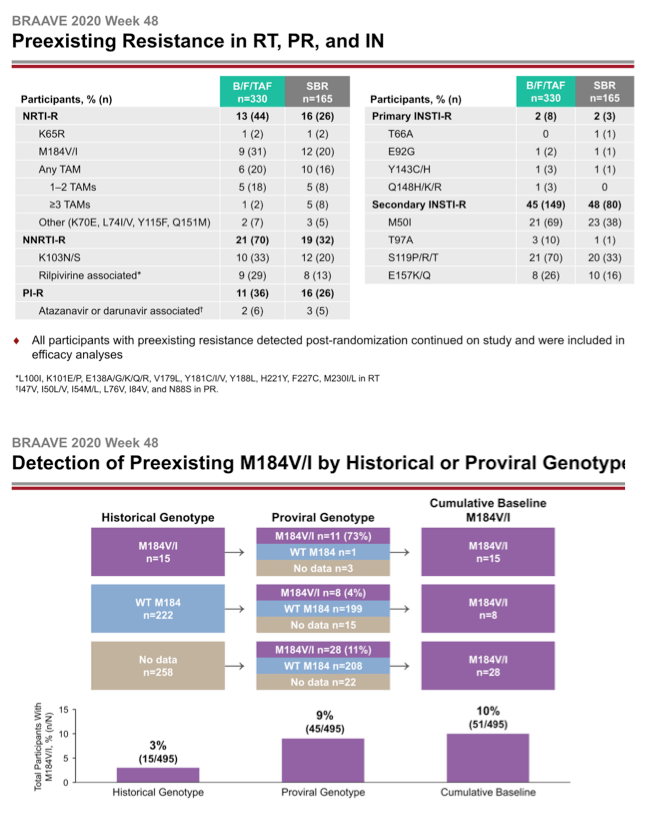
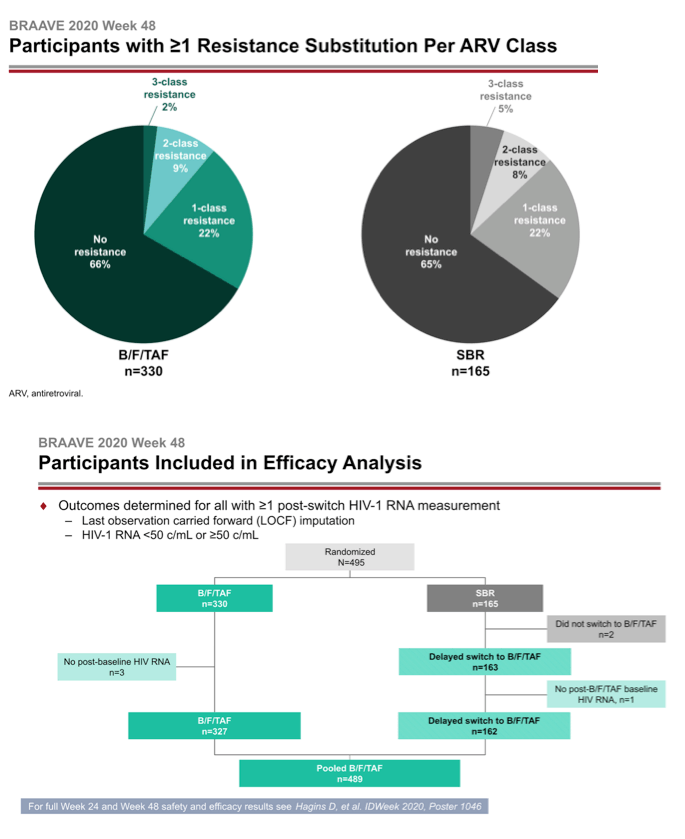
The investigators randomized 330 people to switch to B/F/TAF and 165 to continue their current regimen. At that point 14% in the whole group had primary NRTI mutations, 21% had NNRTI mutations, and 13% had PI mutations. One in 10 people had the M184V/I mutation, 7% had TAMs, and 2% had primary integrase inhibitor mutations.
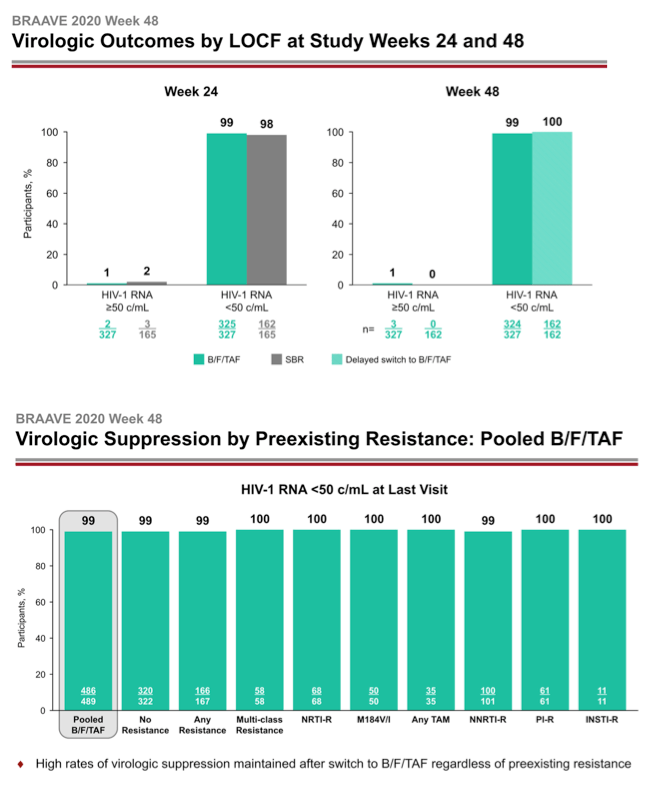
After 24 weeks of treatment 162 people who stayed with their current regimen switched to B/F/TAF. They joined 327 people from the original switch group to make up the 489 people analyzed at week 48. At week 24 a last-observation-carried-forward (LOCF) analysis determined that 99% randomized to B/F/TAF and 98% randomized to stick with their existing regimen had a viral load below 50 copies, a result establishing the noninferiority of B/F/TAF to continued treatment with a suppressive regimen. After 48 weeks 99% randomized to B/F/TAF and 100% who switched to B/F/TAF at week 24 had a sub-50-copy viral load in an LOCF analysis.
A week-48 analysis of viral loads below 50 copies found that every subgroup analyzed had a response rate of 99% or 100%: the 489-person pooled B/F/TAF group (99%), 322 people with no detected resistance (99%), 167 with any resistance (99%), 58 with multiclass resistance (100%), 68 with NRTI mutations (100%), 50 with the M184V/I mutation (100%), 35 with any thymidine analog mutation (100%), 101 with NNRTI mutations (99%), 61 with PI mutations (100%), and 11 with integrase inhibitor mutations (100%).
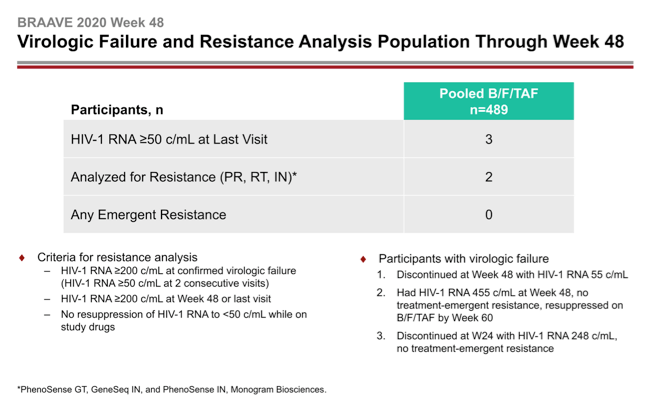
Three people in the pooled B/F/TAF group had a viral load above 50 copies at their last viral load measure. Two had enough virus sampled to test for resistance in reverse transcriptase, protease, and integrase. Neither of these 2 had a resistance mutation emerge during through 48 weeks.
Gilead Sciences researchers noted that adding the 50 people with a preexisting M184V/I mutation in this trial to those in other trials of people switching to B/F/TAF yielded a group of 182 people, 179 (98%) of whom maintained HIV control with no newly emerging mutations.
References
1. Andreatta K, D'Antoni ML, Chang S, et al. Preexisting resistance and week 48 virologic outcomes after switching to B/F/TAF in African American adults with HIV. IDWeek 2020, October 22-25, 2020. Abstract 109.
2. ClinicalTrials.gov. Study to evaluate switching from a regimen of two nucleos(t)ide reverse transcriptase inhibitors (NRTI) plus a third agent to a fixed dose combination (FDC) of bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF), in virologically-suppressed, HIV-1 infected African American participants (BRAAVE 2020). ClinicalTrials.gov identifier NCT03631732. https://clinicaltrials.gov/ct2/show/NCT03631732


|
| |
|
 |
 |
|
|