| |
Coronavirus puts drug repurposing on the fast track
|
| |
| |
Existing antivirals and knowledge gained from the SARS and MERS outbreaks gain traction as the fastest route to fight the current coronavirus epidemic.
27 February 2020
https://www.nature.com/articles/d41587-020-00003-1
Nature Biotechnology
Last month, Hangzhou-based Ascletis Pharma applied to the Chinese authorities to test two HIV protease inhibitors (ritonavir and ASC09) in clinical trials to treat COVID-19, the illness caused by the new coronaviÅrus (Table 1). And Suzhou-based BrightGene Bio-Medical Technology announced in early February that it would begin to manufacture Gilead Sciences' remdesivir (GS-5734), a broad-spectrum investigational antiviral, as a treatment for coronavirus infection. Remdesivir, originally developed to treat Ebola virus and then dropped, will also be tested by Gilead in partnership with Chinese health authorities in randomized, controlled trials. "The general genomic layout and the general replication kinetics and the biology of the MERS, SARS and [SARS-CoV-2] viruses are very similar, so testing drugs which target relatively generic parts of these coronaviruses is a logical step," says Vincent Munster, chief, Viral Ecology Unit, US National Institute of Health. Testing therapies approved for other indications also makes senses, as these drugs are already mass produced and available on a large scale.
From the start of the COVID-19 outbreak, medical practitioners have followed China's guidelines set up in January and treated hospitalized patients with α-interferon combined with the repurposed drug Kaletra, an approved cocktail of the HIV protease inhibitors ritonavir and lopinavir. The World Health Organization has noted that this combination could provide some clinical benefit. Kaletra, manufactured by AbbVie, is also being tested in other combinations, with repurposed drugs known to target parts of the replication machinery of other viruses that are similar to those in SARS-CoV-2 - for instance, with the guanosine analog and RNA synthesis inhibitor ribavirin, with reverse transcriptase inhibitors (emtricitabine/tenofovir alafenamide fumarate) or with Moscow-based Pharmstandard's membrane fusion inhibitor umifenovir. Umifenovir is also in trials as a single agent.
SARS-CoV-2 is an enveloped, positive-sense, single-stranded RNA β-coronavirus similar to the severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) viruses. Potential antiviral targets encoded by the viral genome include non-structural proteins (e.g., 3-chymotrypsin-like protease, papain-like protease, RNA-dependent RNA polymerase and its helicase), structural proteins (e.g., the capsid spike glycoprotein) and accessory proteins. Kaletra is thought to inhibit the 3-chymotrypsin-like protease of the SARS and MERS coronaviruses and was associated with improved clinical outcomes in a trial against SARS. Ascletis also reported that a patient with COVID-19 improved rapidly when given this HIV protease inhibitor combination.
But Erik De Clercq, of the Rega Institute for Medical Research in Leuven, Belgium, says that in searching for or designing effective drugs against COVID-19: "We should stay away from antivirals known to be acting at targets not playing a role in the replication of coronaviruses." Such drugs include penciclovir, which is targeted at the herpesvirus DNA polymerase, and lopinavir/ritonavir, which are targeted at the HIV protease. Instead, he would favor targeting a virus-specific protein such as the RNA-dependent RNA polymerase, noting that coronaviruses do not contain or use a reverse transcriptase. George Painter, president of the Emory Institute for Drug Development, Emory University, is also cautious about the HIV protease inhibitor strategy. "It's probably a long shot to go for drug repurposing activity against the coronavirus using HIV drugs; these were protease inhibitors that were designed specifically for HIV," he says.
Nonetheless, several clinical trials of Kaletra are underway, either alone or with various combinations of interferons, guanosine-analog RNA synthesis inhibitors, reverse transcriptase inhibitors or influenza drugs, such as baloxavir marboxil, oseltamivir and umifenovir (Table 1). These trials are anticipated to read out from the end of May onwards.
Painter is more optimistic about Gilead's investigational drug remdesivir, a nucleotide analog antiviral, that blocks the RNA polymerase of the Ebola virus and so prevents replication. The thinking behind repurposing remdesivir is that its broad antiviral activity may render it effective against SARS-CoV-2. Indeed, remdesivir is in two clinical trials that began early February, with an estimated completion date of early April. "Remdesivir has quite high efficacy across all different coronaviruses and therefore it is one of the prime candidates to start being tested," says Munster. The World Health Organization's R&D Blueprint report released at the end of January considered remdesivir the most promising candidate to treat COVID-19, on the basis of its broad-spectrum activity, in vitro and in vivo data for coronaviruses and clinical safety from Ebola virus disease trials.
In vitro studies published in January have shown remdesivir to be active against a clinical isolate of SARS-CoV-2. Experimental data in mice infected with the related MERS virus also showed the drug was better than a combination of lopinavir/ritonavir and interferon beta in improving lung function. And the first patient in the United States with confirmed COVID-19 improved after being treated for one day with remdesivir, although this could not be directly attributed to the drug's effect. Since then, remdesivir has been shown to reduce the severity of disease, virus replication and damage to the lungs in a non-human primate model of MERS.
"Broad-spectrum agents are ideally suited for outbreak situations where we don't entirely know what we are dealing with in terms of pathogens," says Bryan Mounce, assistant professor, Department of Microbiology and Immunology, Loyola University Chicago. "Although we might not understand all the mechanisms underlying their antiviral activity, it is important that they have as few side effects as possible," he adds. A second broad-spectrum antiviral in trials (Table 1) is Tokyo-based Toyama Chemical's RNA polymerase inhibitor favipiravir, approved for use against influenza A and B. In an in vitro study, this compound did not show strong activity against a clinical isolate of SARS-CoV-2.
Another HIV protease inhibitor, Janssen's Prezcobix (darunavir and the boosting agent cobicistat), is also under evaluation. At the end of January, Janssen shipped Prezcobix to China for in vitro testing. In a statement to Nature Biotechnology, the company said, "We are aware of anecdotal reports of darunavir potentially having antiviral activity against COVID-19. The company has no in vitro or clinical data to support the use of darunavir as a treatment for COVID-19." The recipients of the shipments have registered a clinical trial to test Prezcobix in combinations.
At least ten clinical trials are testing chloroquine, approved as an antimalarial and autoimmune disease drug. In vitro, the endosomal acidification fusion inhibitor blocked infection of a clinical isolate of SARS-CoV-2.
Most of the drugs in clinical trials (Table 1) inhibit key components of the coronavirus infection lifecycle. These include viral entry into the host cell (blocked by umifenovir, chloroquine or interferon), viral replication (blocked by lopinavir/ritonavir, ASC09 or darunavir/cobicistat, which inhibit the 3C-like protease (3CLpro)) and viral RNA synthesis (inhibited by remdesivir, favipiravir, emtricitabine/tenofovir alafenamide or ribavirin). The genomic sequence of the SARS-CoV-2 suggests that there is a high level of sequence similarity between the SARS-CoV-2, SARS and MERS proteins involved in the replication cycle.
Targeting viral cellular entry via the spike glycoprotein, which mediates the virus-cell receptor interaction, is another option for repurposing. SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) and the cellular protease transmembrane protease serine 2 (TMPRSS2) to enter target cells. The marketed TMPRSS2 inhibitor camostat mesylate blocked cellular entry of the SARS-CoV-2 virus, according to an unpublished preprint. And the Janus-associated kinase (JAK) inhibitor Olumiant (baricitinib), approved for rheumatoid arthritis, was identified using machine learning algorithms on the basis of its inhibition of ACE2-mediated endocytosis. Another JAK inhibitor, Jakafi (ruxolitinib), is in trials (combined with mesenchymal stem cell infusion) for COVID-19.
The release earlier this month of the high-resolution crystal structure of 3CLpro (Protein Data Bank identifier 6LU7) and a Science paper describing the 3.5-Å-resolution structure of the viral spike S protein in pre-fusion conformation should also expedite drug discovery efforts. Researchers who determined the 3CLpro structure reportedly used it to screen for repurposed drugs, identifying the protease inhibitors saquinavir, indinavir, lopinavir and ritonavir, the proteasome inhibitor carfilzomib, two respiratory syncytial virus drugs, a schizophrenia medication and an immunosuppressant. Also on the basis of the 3CLpro structure, machine learning algorithms (generative deep learning) generated several novel potential inhibitors. Turning these into drugs would require a very lengthy drug development process, which would probably be longer than the current outbreak. One of the authors of the preprint, Alex Zhavoronkov, CEO at Insilico Medicine, Hong Kong, suggests that finding similarities between these novel structures and known drugs could be an option for repurposing - and here the speed of machine learning searches could be particularly useful in epidemic settings.
But even without SARS-CoV-2 structures one can do virtual screening. "For a first approximation, one can work with the enzyme structures that we have from SARS coronavirus," says Rolf Hilgenfeld, Institute of Biochemistry, University of Lubeck, Germany. "Of course, there are some differences, but most of these won't affect the binding sites for inhibitors to the substrate," he says.
Even though virtual screening makes it possible to discover molecules relatively quickly, these compounds still need to be experimentally tested - for example, against the virus in cell culture - before any further steps can be taken. "Unfortunately, with previous outbreaks, there is the experience that not all of these studies are very sound, they have been done under tight pressure, and so one has to be a little bit cautious about the results," says Hilgenfeld.
Targeting viral replication with drugs such as remdesivir should prevent asymptomatic, mild or moderate COVID-19 cases from progressing to severe disease. Yet current drugs in trials might not be enough for those who are sickest. "Typically those presenting themselves at hospitals will already have severe disease, which is associated with pneumonia. Here, targeting viral replication might take away the virus but not the damage that is very likely due to the immune response of the patient," says Munster.
The repurposed pipeline is finite, so it's almost certain that novel as well as repurposed drugs will be needed in the fight against COVID-19. Those that hold the most promise, Painter notes, are those antivirals with a breadth of activity against not only coronaviruses, but other RNA viruses, such as highly pathogenic Ebola and avian flu viruses.. "If there's any message to be had from the current outbreak, it's that it's almost inevitable it will happen again."
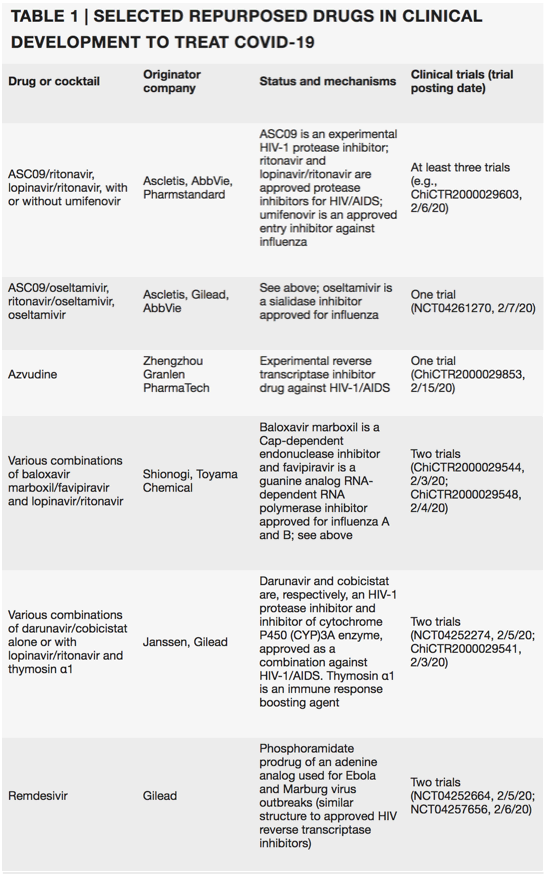
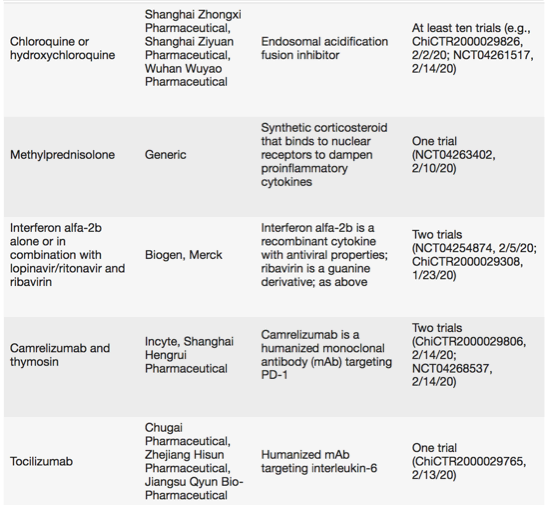
Last search run on 15 February using https://clinicaltrials.gov and http://www.chictr.org.cn. Excludes traditional Chinese medicines and blood-derived products, such as serum from recovered patients and stem cells. All trials are being conducted in China.
|
|
| |
| |
|
|
|