 |
 |
 |
| |
THE IMPACT OF THE COVID-19 PANDEMIC ON HCV TESTING AND TREATMENT IN THE UNITED STATES - Between February 2020 and March 2020, the predicted number of tests dropped significantly by 33% but started to improve; Treatment Initiation dropped by 31%
|
| |
| |
AASLD 2021
Martin Hoenigl1, Daniela Abramovitz1, Ricardo Flores1, Natasha Martin1,2 and Nancy Reau3, (1)Division of Infectious Diseases and Global Public Health, University of California San Diego, (2)Population Health Sciences, University of Bristol, United Kingdom, (3)Hepatology, Rush University Medical Center
Background: Recent reports indicated declines in HCV testing during the first half of 2020 in the United States due to COVID-19, but it is unclear whether a longer-term impact on HCV testing and treatment initiations was observed. The objective of this study was to investigate the nationwide impact of the pandemic on HCV testing and treatment in the United States through the end of 2020.
Methods: We obtained monthly state-level volumes of HCV Ab testing and HCV treatment initiation, stratified by age, spanning two years from January 2019 until December 2020 from two large national labs (Quest and LabCorp). We performed segmented regression analysis by obtaining the estimated numbers of tests and treatments for each state from a mixed effects negative binomial model with Month as the main fixed predictor, and State as a random intercept. The predicted values from the model were aggregated by calculating the sum of the predicted number of tests or treatment initiations for each month and then aggregated values were regressed on month. Maximum likelihood estimation was used. Months from 1/2019 to 2/2020 (Months 1-14 in Figure) were designated the 'pre-COVID period' and 3/2020 to 12/2020 (Months 15-24) were designated the 'COVID period'.
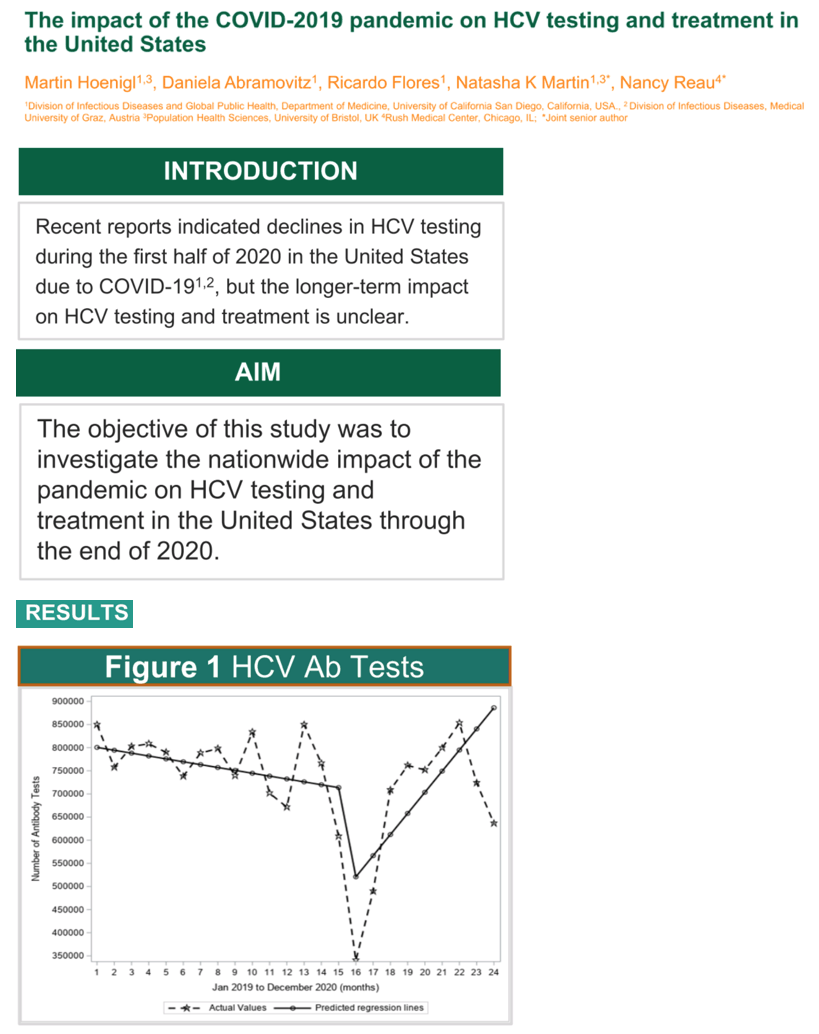
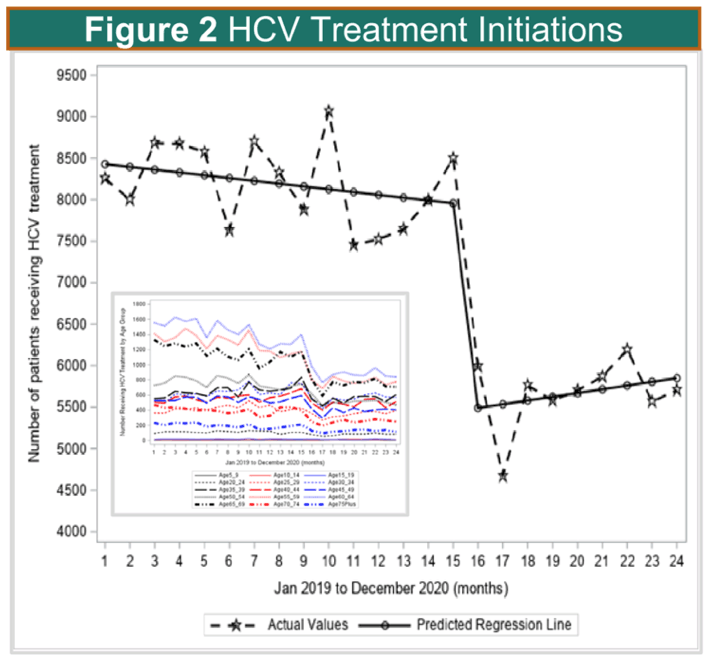
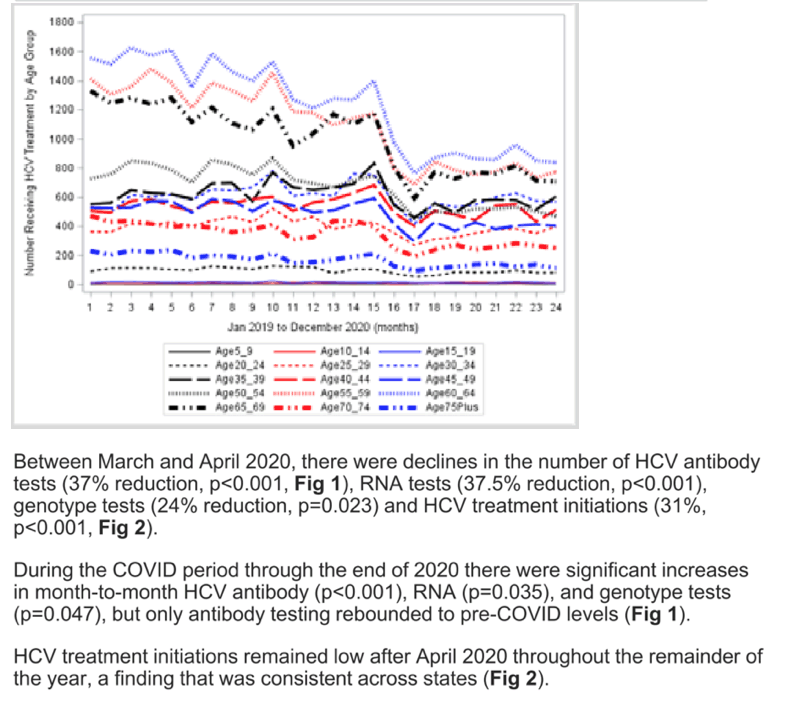
Results: During the pre-COVID period, the monthly number of HCV antibody tests in the US remained relatively stable with slight declines (p=0.712). Between February 2020 and March 2020, the predicted number of tests dropped significantly by 33% (p=0.004; similar trend in males and females and by state). During the COVID period through the end of 2020 (compared to the month-to-month trend before the COVID period) there was a significant increase in month-to-month testing (p=0.008; Figure 1). During the pre-COVID period, HCV treatment initiations were stable, but dropped significantly from March 2020 to April 2020 by 31% (p<=0.0001; Figure 1) and remained low throughout the remainder of the year during the COVID period. In sub-analyses, there were similar trends in HCV treatment declines across age groups and by state.
Conclusion: Monthly HCV Ab testing and treatment volumes dropped by over 30% during the COVID pandemic, but while HCV Ab testing rebounded to pre-COVID levels by the end of 2020, HCV treatment rates remained at a constant low level through the end of the year. Efforts should be made to link the HCV Ab positive patients to treatment, and revitalize HCV treatment engagement by health care providers.
|
| |
|
 |
 |
|
|