 |
 |
 |
| |
USE OF SOFOSBUVIR/VELPATASVIR IN TEST AND TREAT STRATEGY IN FRANCE:
SNAPSHOT ANALYSIS OF PROSPECTIVE COHORT STUDY HELIOS 3
|
| |
| |
Denis Ouzan1,2, Jean Pierre Bronowicki3, Christophe Renou4, Laure Ekrief5, Ghassan Riachi6, Michel Antoni7, Dan Pospait Sr.8, Alexandra Heurgue9, Hatem Salloum10, Jean Jacques Meurisse11, Laurent Roudiere12, Laurent Cuissard13, Vincent Leroy14, Joseph Koffi15, Philippe Gouiry16,17, Matthieu Chardon18, Priscila Pajaud Passe-Coutrin19, Juliette Foucher20, Magdalena Meszaros21, Sylvie Balteau22, Patrick Vogt23, Celine Douzon24, Moana Gelu-Simeon25, Etienne Hieguel26, Yavor Delchef12, Anne Strateman27, Olivier Lada28, Nathalie Renaud29, Isabelle Fouchard Hubert30, Laura Telep31, Christophe Hezode32, Laurent Cattan12 and Stanislas Pol33,
(1)Institut Arnaud Tzanck, Saint Laurent Du Var, France, (2)Hepatology, Institut Arnaud Tzanck Saint Laurent Du Var, (3)Hepatology, Nancy University Hospital, (4)Hepato-Gastroenterology, CH Hyères, (5)CHU Tours, (6)CHU Rouen, (7)Hepatogastroentérologie, Hopital D'orange,(8)Hepato-Gastroenterology, Hôpital Bichat-Claude Bernard,Paris, (9)Department of Hepatology and Digestive Diseases,Robert-Debré University Hospital, (10)Hepatology, MeauxHospital, (11)Hepatology, Bourg En Bresse Hospital, (12)General Practice of Medecine, (13)Hepatology, Clinique LesOrchidées, (14)Hepatology, Henri Mondor Hospital, Creteil,France, (15)Perigueux Hospital, (16)Prison of Beziers,(17)Béziers Correctional Center, (18)Accueil LiaisonsToxicomanie Association (ALT), (19)Chemin Vert_ Medical House, (20)Hepatology, Hôpital Du Haut-Lévêque, (21) Montpellier Hospital, (22) Csapa Les Wads, (23)Mulhouse Jail, (24)Saint Roch Hospital, (25)Hepatology, Guadeloupe University Hospital, (26)Jury Les Metz Hospital, (27)Prison of Albi, (28)Medical Affairs, Gilead Sciences, Inc., (29)Clinical Operation, Gilead Sciences France, (30)Angers University Hospital, (31)Gilead Sciences US, (32)Medical Affair, Gilead Sciences, Inc., (33)Hepatology, Hopital Cochin
Background: In France, prescribing authorization for hepatitis C virus (HCV) treatment has been expanded to primary care physicians (including those in addictions centers, jails, and psychiatric centers). Patient management includes a Test and Treat approach (TnT) to expedite treatment initiation (applicable to all prescribers) and a pathway dedicated to severe patients followed-up by specialists. This is a descriptive analysis of the study population to assess the impact of prescription expansion on Sofosbuvir/Velpatasvir (SOF/VEL) usage in a TnT strategy in real life practice settings.
Methods: Included patients are HCV-infected adults following SOF/VEL-based regimens prescribed by primary care physicians and specialists. Data collected from available information in medical records include patient characteristics and number of days between positive PCR/fibrosis assessment and start of therapy. Qualitative variables are described as number and percentage (%); quantitative measures as mean (standard deviation; SD) or median (range) as indicated.
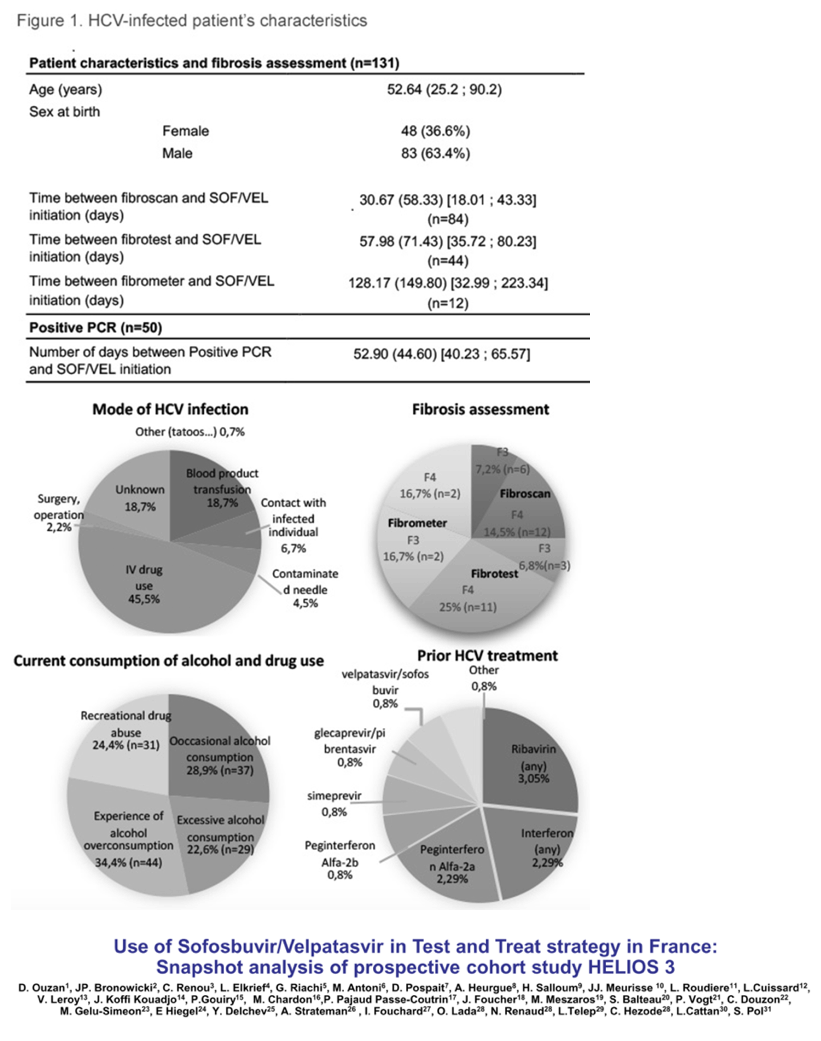
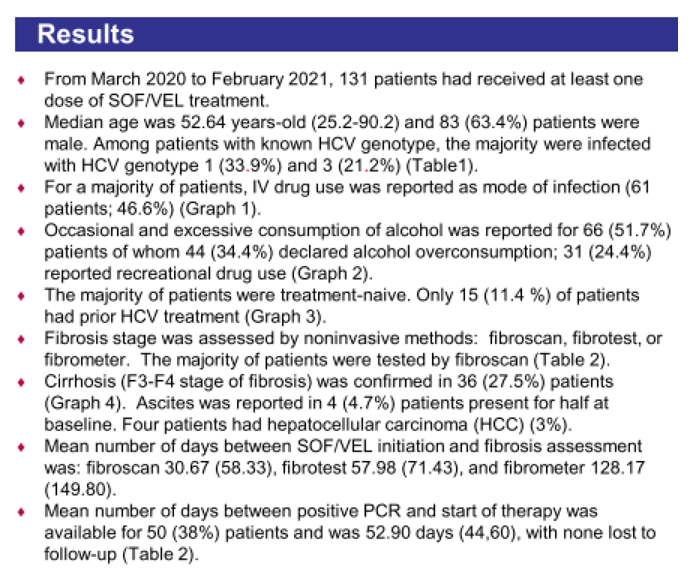
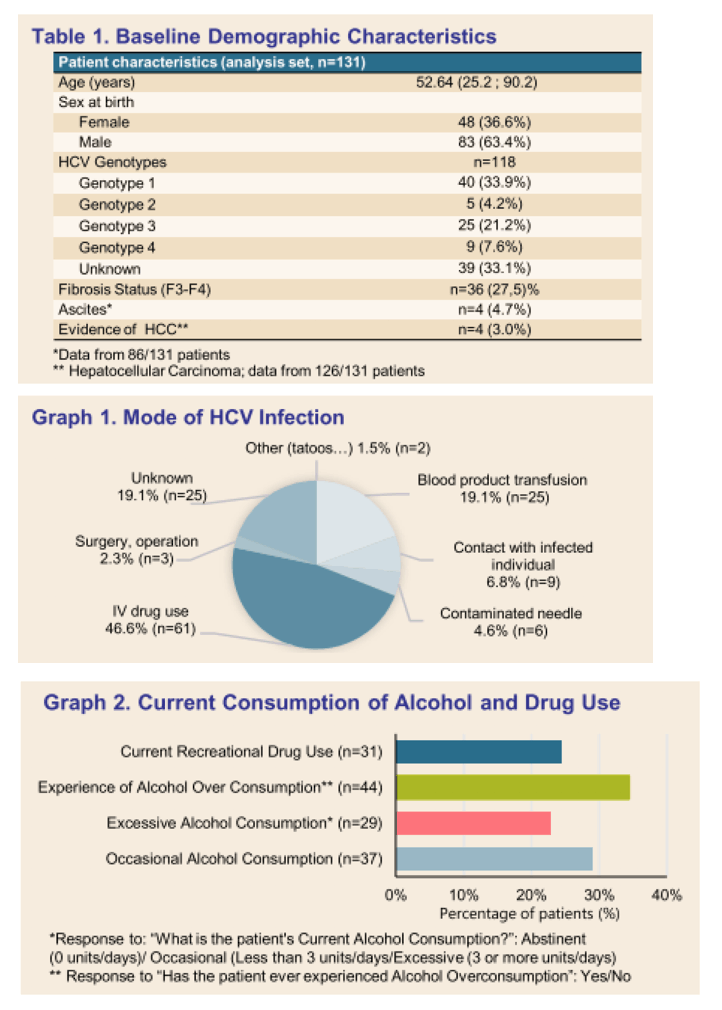
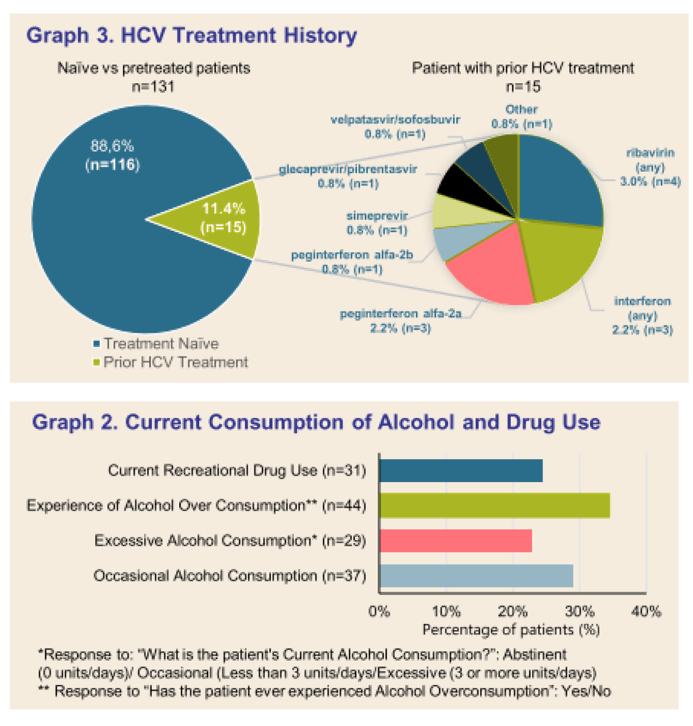
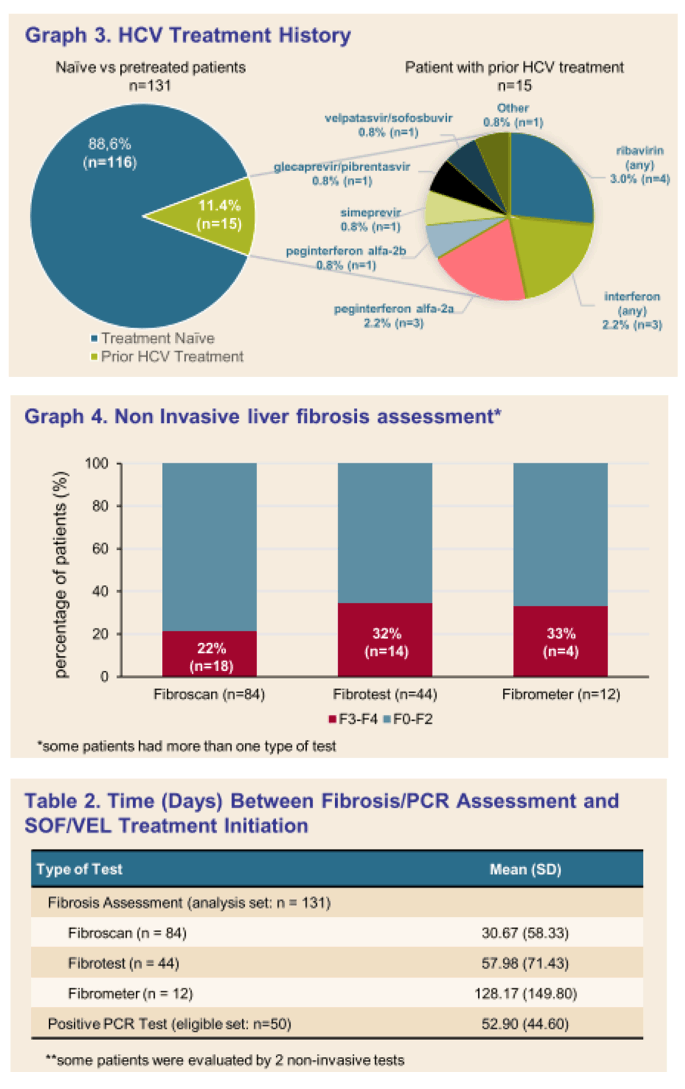
Results: (Figure) From March 2020 to February 2021, 131 patients had received at least one dose of SOF/VEL treatment. Median age was 52.64 years-old (25.2-90.2), 83 (63.4%) patients were male. Occasional and excessive consumption of alcohol was reported for 66 (51.7%) patients of whom 44 (34.4%) declared alcohol overconsumption; 31 (24.4%) reported recreational drug use. IV drug was reported as mode of infection for 61 (45.5%) patients. Only 10 (7.6 %) patients had prior HCV treatment. Eighty-four (65%) patients had fibroscan, 44 (34%) had fibrotest, and 12 (9.3%) had fibrometer; Cirrhosis (F3-F4 stage of fibrosis) was confirmed in 36 (27.5%) patients. Ascites was reported in 4 (4.7%) patients, ongoing for half at study inclusion. Mean number of days between SOF/VEL initiation and fibrosis assessment was: fibroscan 30.67 (58.33), fibrotest 57.98 (71.43), and fibrometer 128.17 (149.80). Median number of days between positive PCR and start of therapy was available for 50 (38%) patients and was 38.50 days (0-170), and none was lost to follow-up. Complementary FIB4 score analysis is ongoing.
Conclusion: This snapshot analysis describes a French study population benefiting from SOF/VEL TnT in real life after treatment prescription expansion; difficult to treat patients (IV drug users, Alcoholics, Severe Fibrosis) could have access to SOF/VEL therapy. Treatment simplification and prescription expansion create an opportunity to speed access to treatment and avoid patient attrition. Patient's pathway may be optimized according to fibrosis assessment step.

|
| |
|
 |
 |
|
|