 |
 |
 |
| |
A COMMUNITY-BASED TRIAL OF HEPATITIS C TREATMENT AT POINT OF DIAGNOSIS FOR MARGINALIZED POPULATIONS: PRELIMINARY RESULTS FROM THE NO ONE WAITS (NOW) STUDY
|
| |
| |
AASLD: HCV-RNA VIRAL LOAD FINGERSTICK ASSAY AS A SIMPLIFIED STRATEGY FOR SCREENING AND LINKAGE TO CARE OF PEOPLE WHO USE DRUGS ATTENDING ITALIAN ADDICTION TREATMENT CENTRES: A PILOT PROJECT - (11/15/21)
AASLD: Rapid Hepatitis C Treatment Initiation in Young People Who Inject Drugs: Final Results from the HCV-Seek, Test & Rapid Treatment (HCV-ST&RT) Randomized Pilot Clinical Trial - (11/15/21)
AASLD 2021 Nov 12-15 Meghan Morris UCSF

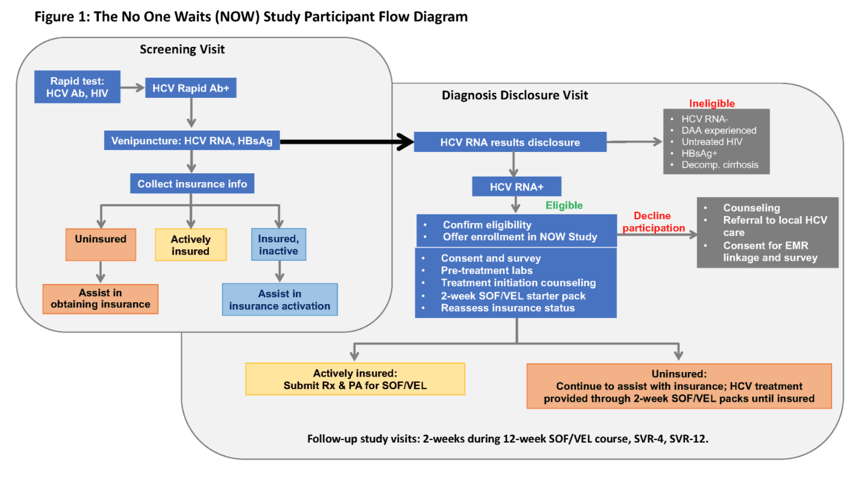
Background: A principal barrier to hepatitis C virus (HCV) treatment is linkage to care upon diagnosis, particularly for marginalized populations. Pairing community-based HCV testing services with low-threshold treatment eliminates the need for patients to navigate the medical system.
Methods: We conducted a single-arm trial in an urban US community setting to assess the acceptability, feasibility, and effectiveness of delivering HCV treatment at the point of HCV RNA positive diagnosis disclosure. Street-outreach recruitment targeted people experiencing homelessness and injecting drugs for rapid HCV antibody (anti-HCV) testing followed by confirmatory HCV RNA testing if positive (Figure). At HCV RNA result disclosure, enrolled participants were given 2-weeks of study-provided SOF/VEL and transitioned to insurance-provided SOF/VEL upon authorization. Based on enrollment to date, we estimated (i) time from HCV RNA disclosure to treatment initiation, (ii) proportion who completed treatment, and (iii) proportion who attained SVR-12.
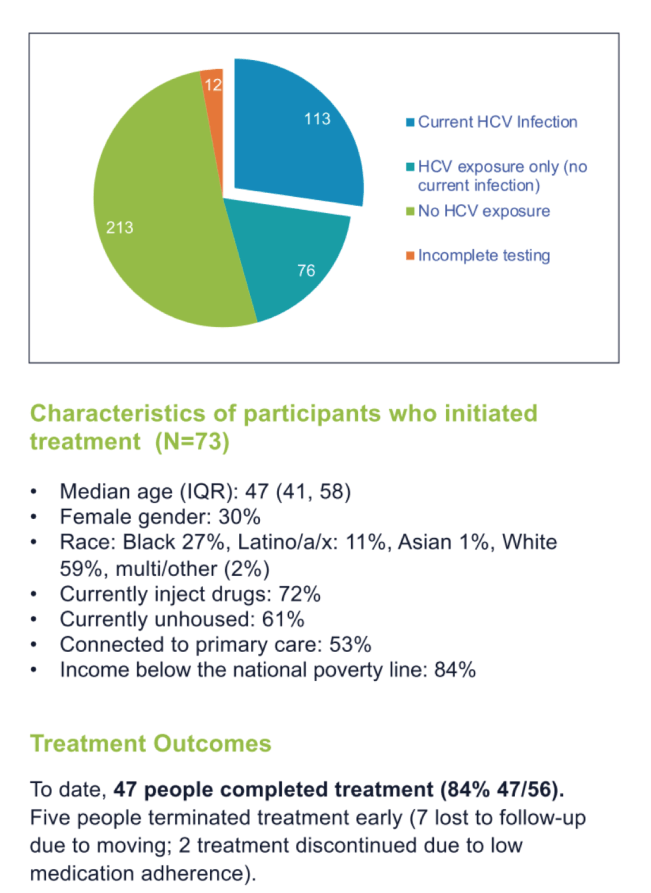
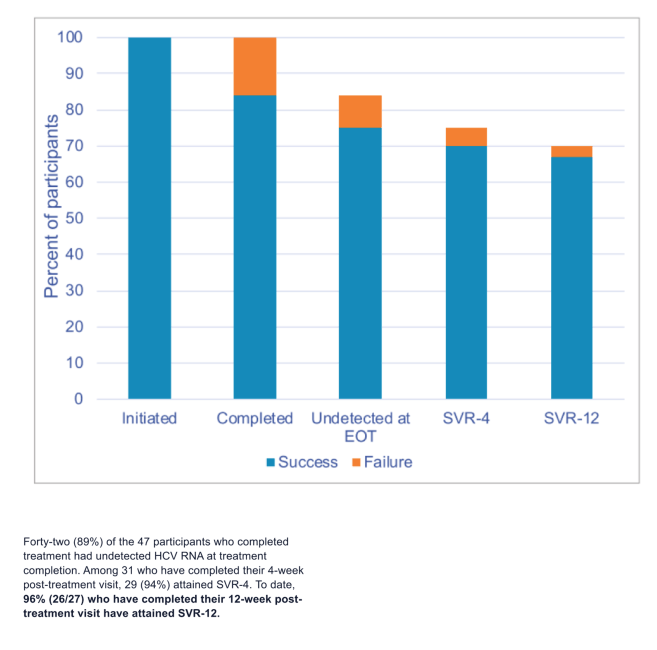
Results: Between July 2020 and May 2021, 300 individuals were screened; to date, 52 (17%) have tested anti-HCV positive and RNA negative and 70 (23%) anti-HCV and HCV RNA positive. Fifty-eight of the 70 HCV RNA positive (83%) returned for diagnosis disclosure and were eligible to enroll. To date, 57 (98%) initiated treatment upon diagnosis disclosure (median age: 47 years [IQR: 41, 58]). Forty (70%) were injecting drugs, 35 (61%) were unhoused, and 30 (52.6%) had a primary care provider. Thirty-two (87% 32/37) have completed treatment, all of whom had undetectable HCV RNA upon completion. To date, lost to follow-up occurred for 3 patients (due to moving) and treatment termination due to low medication adherence occurred for 2. Ninety-six percent (23/24) have attained SVR-4 and 100% (14/14) have attained SVR-12.
Conclusion: Preliminary results from our trial of people experiencing homelessness and currently injecting drugs indicate that initiation at the time of diagnosis is acceptable, feasible, and results in high treatment completion and SVR.

|
| |
|
 |
 |
|
|