 |
 |
 |
| |
PROTEASE INHIBITORS (PI) IN TREATMENT OF HEPATITIS C
AND THE RISK OF HEPATIC DECOMPENSATION:
A META-ANALYSIS OF PI REGIMEN SAFETY
|
| |
| |
AASLD 2021 Nov 1215
Hydar El Jamaly, Department of Gastroenterology and Hepatology, Westmead Hospital, Jacob George, Storr Liver Centre, Westmead Institute for Medical Research, University of Sydney and Westmead Hospital and Mark Douglas, The Westmead Institute for Medical Research
Background: The use of protease inhibitors (PI) is contraindicated in patients with HCV infection and decompensated cirrhosis and must be used with caution in compensated cirrhosis. We aimed to undertake a meta-analysis of the risk of hepatic decompensation in patients with hepatitis C treated with a protease inhibitor containing regimen.
Methods: A comprehensive search was conducted for any publication year until June 2021 including grey literature. Clinical trials and real-world two-armed active comparator studies were included with special population groups such as HBV, HIV co-infected cohorts and chronic renal failure patients included. A MeSH term search was conducted for protease inhibitors by category and keyword search of individual drug generic names using Medline, Embase and Cochrane Library. Hepatic decompensation was defined as the onset of confirmed variceal bleed, ascites, SBP, hepatic encephalopathy or hepatorenal syndrome. The minimal accepted PI regimen was 4 weeks. The risk of decompensation was calculated using odds ratios and 95% confidence intervals using a random effects model.
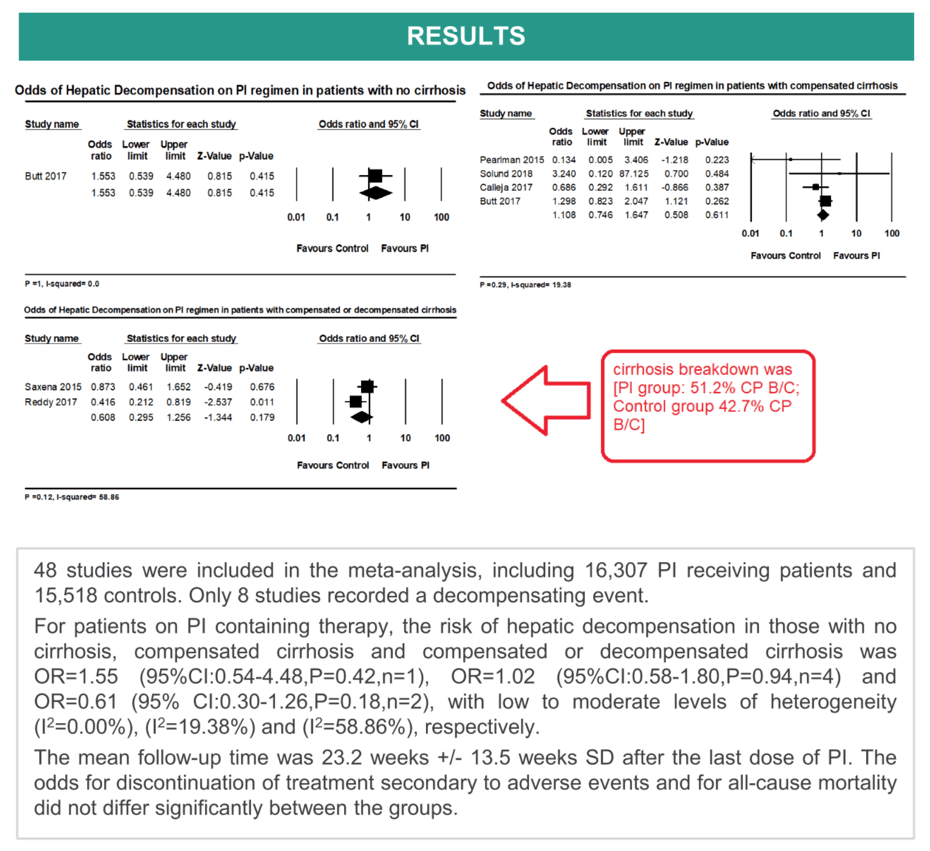
Results: 48 studies were included in the meta-analysis, including 16,307 PI receiving patients and 15,518 controls. Only 8 studies recorded a decompensating event and no studies were performed purely on patients with decompensated cirrhosis. For patients on PI containing therapy, the risk of hepatic decompensation in those with no cirrhosis, compensated cirrhosis and compensated or decompensated cirrhosis was OR=1.55 (95%CI:0.54-4.48,P=0.42,n=1), OR=1.02 (95%CI:0.58-1.80,P=0.94,n=4) and OR=0.61 (95% CI:0.30-1.26,P=0.18,n=2), with low to moderate levels of heterogeneity (I2=0.00%), (I2=19.38%) and (I2=58.86%), respectively. In the two studies recruiting patients with compensated or decompensated cirrhosis, the cirrhosis breakdown was [PI group: 51.2% CP B/C; Control group 42.7% CP B/C]. The mean follow-up time was 23.2 weeks +/- 13.5 weeks SD after the last dose of PI. The odds for discontinuation of treatment secondary to adverse events and for all-cause mortality did not differ significantly between the groups.
Conclusion: This meta-analysis suggests there is no statistically significant difference in the odds of hepatic decompensation on PI therapy in all grades of cirrhosis/fibrosis. A larger true effect is expected to be mitigated by the low event rate of hepatic decompensation and careful patient selection.


|
| |
|
 |
 |
|
|