 |
 |
 |
| |
HEPATITIS C TREATMENT IN THE UNITED STATES, 2014-2020 numbers of
treated is "below that needed to ensure elimination in USA by 2030,
increased screening needed, remove all rstrictions"
|
| |
| |
AASLD 2021 Nov 12-15
Eyasu H. Teshale, Henry Roberts, Neil Gupta and Ruth Jiles, Division of Viral Hepatitis, Center for Disease Control and Prevention
Background: In the United States, hepatitis C virus (HCV) is a leading cause of chronic liver disease, cirrhosis, and liver cancer. Monitoring the number and characteristics of HCV-infected persons treated is important to assess progress towards elimination of hepatitis C.
Methods: Using IMS Health & Quintiles (IQVIA) data from 2014-2020, we determined the number of HCV-infected persons treated for hepatitis C with direct-acting antiviral (DAA) agents in the United States by year, type of DAA, payer attributes, provider type, US region, and patient demographic characteristics, including sex, age group, birth cohort, and race/ethnicity.
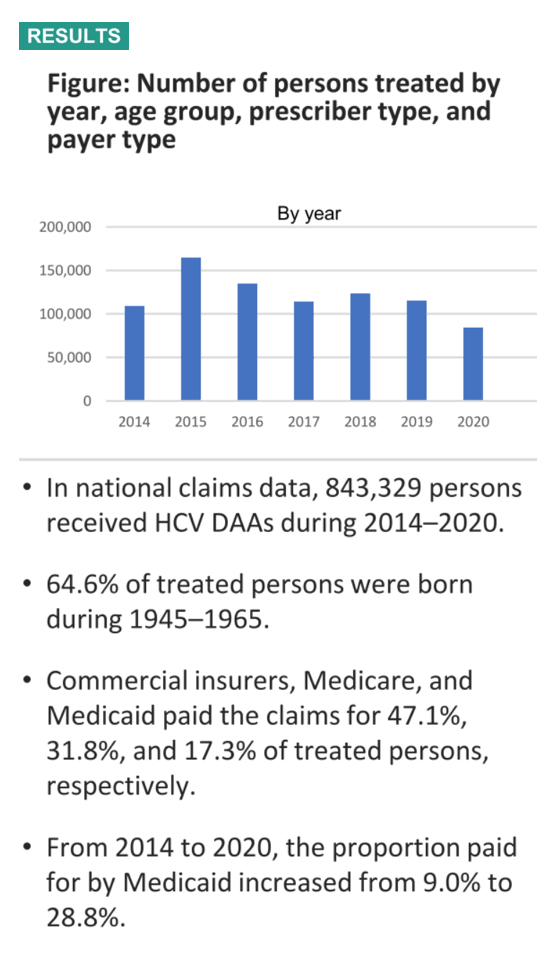
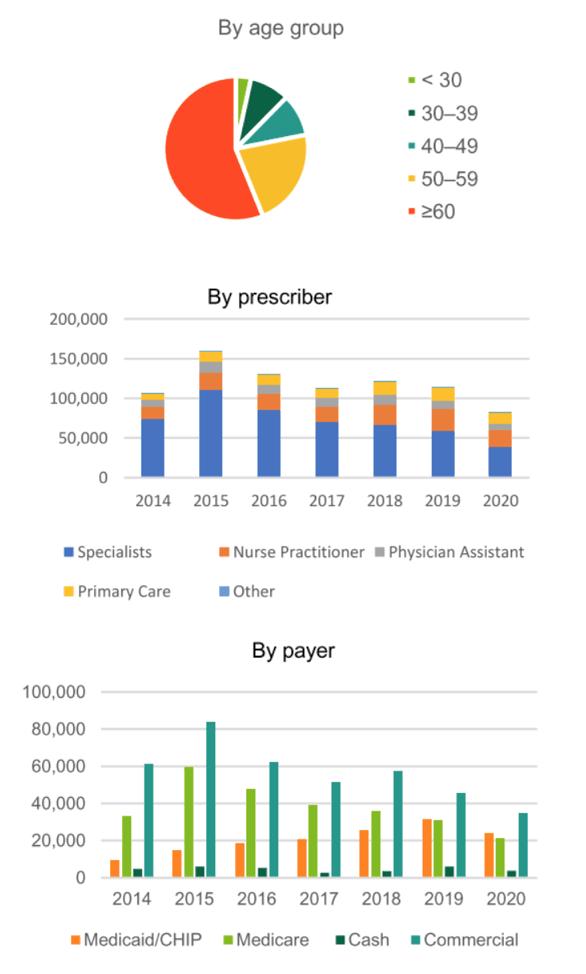
Results: During 2014-2020, there were 843,362 US persons living with hepatitis C who had initiated DAA treatment. Of those receiving DAAs, 59.6% were male, 39.2% were female, and 64.6% were born during 1945-1965. Race/ethnicity information was missing for 66.8% of treated persons, but for those with available data (n=280,003), 68.2% were White, 19.3% were Black, 10.3% were Hispanic, and 2.2% were Asian. From 2014-2020, the proportion of claims paid for by Medicaid increased from 6.6% to 14.6% while claims paid by Medicare decreased from 30.4% to 25.3%. From 2014 to 2020, the annual proportion of HCV DAAs dispensed to patients born during 1945-1965 decreased (73.6% vs. 46.3%) and increased among persons born after 1965 (17.4% vs. 51.3%). Overall, 59.9% of prescribers were specialists. The most commonly dispensed HCV DAA in 2020 was sofosbuvir/velpatasvir (45.8%), followed by glecaprevir/pibrentasvir (44.3%), and ledipasvir/sofosbuvir (6.5%).
Conclusion: During 2014-2020, an average of 120,000 persons with hepatitis C were treated with DAAs annually. This average number of persons living with hepatitis C treated is below the number needed to ensure elimination of hepatitis C as a public health threat in the United States by 2030. Public health interventions such as increased testing and improved linkage to care for groups experiencing disproportionate impact, and removal of restrictions to treatment are critical to improve access to treatment and eliminate hepatitis C as a public health threat in the United States. It is encouraging that the proportion of claims paid by Medicaid is increasing. This change would seem to reflect a gradual reduction in restrictions to DAA prescriptions which has resulted in improved access to treatment of younger populations with less advanced liver disease.


|
| |
|
 |
 |
|
|