 |
 |
 |
| |
ETHNIC VARIATIONS IN CLINICAL PRESENTATION AND TREATMENT
ELIGIBILITY FOR HEPATITIS DELTA VIRUS INFECTION AT A US REFERRAL CENTER
|
| |
| |
AASLD 2021 Nov 12-15
Julian Hercun, David Yardeni, Alexander Yang, Theo Heller and Christopher Koh, Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases
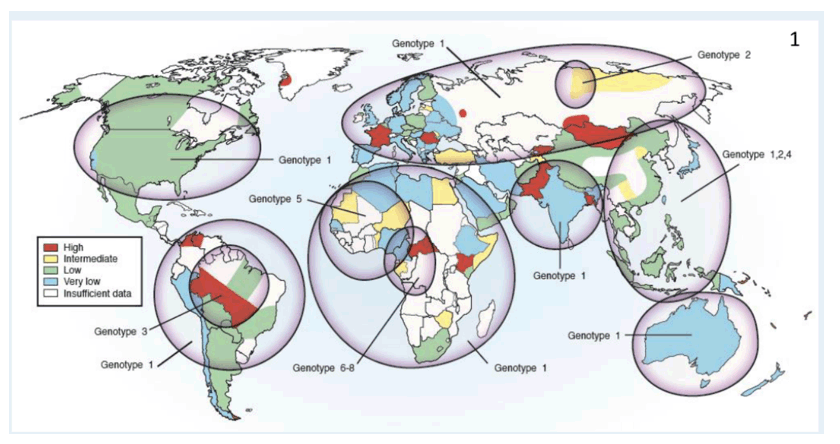
Background: Hepatitis D virus (HDV) infection remains a global public health issue with suboptimal access to treatment amongst ethnic groups. Interferon-based therapies are the only widely available treatments, although their use remains contraindicated in cases of cytopenia (including neutropenia), immunodeficiency or systemic disease. Racial disparities in access and response to interferon therapy of hepatitis C have been reported, however this has not been evaluated for HDV.
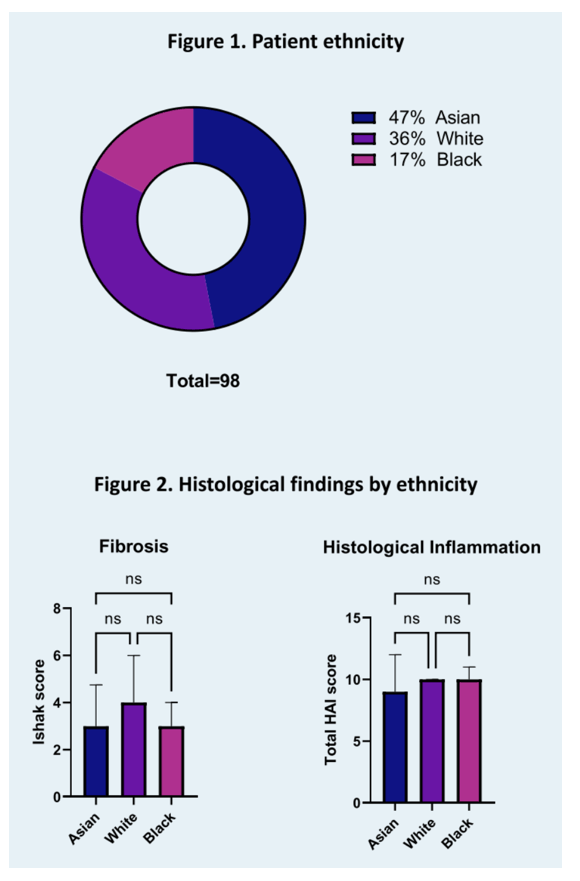
Methods: Retrospective review of all patients referred for a pre-treatment evaluation of chronic HDV at the National Institutes of Health from 2000 to 2021. Concomitant liver disease and HIV infection were excluded. Patients were classified as Asian, White or Black (African) based on their country of origin and comparisons across all groups were performed.
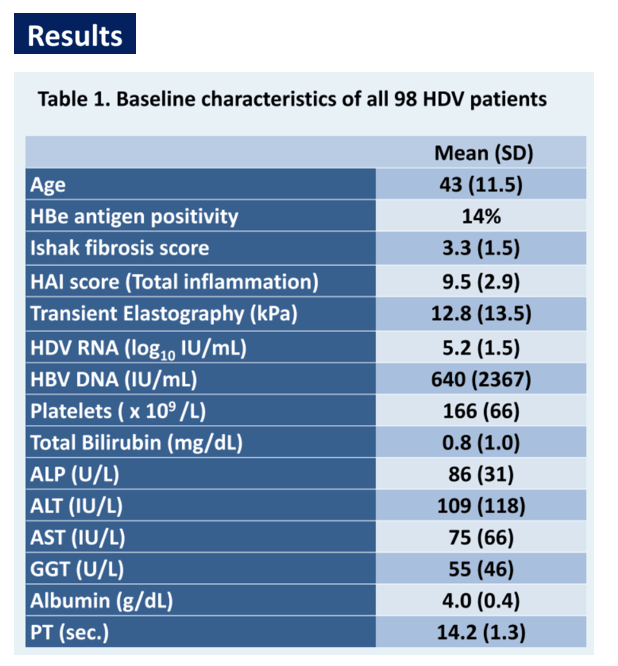
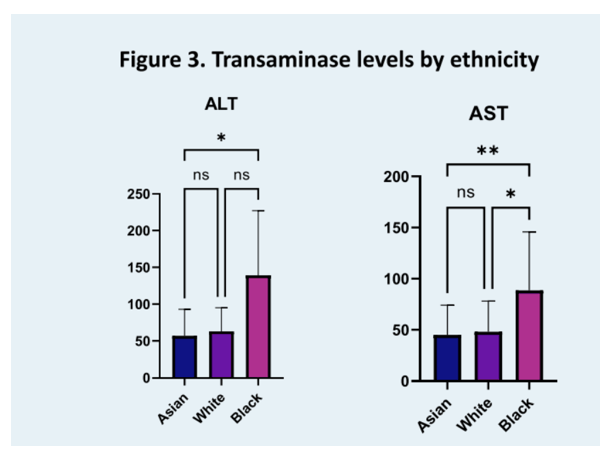
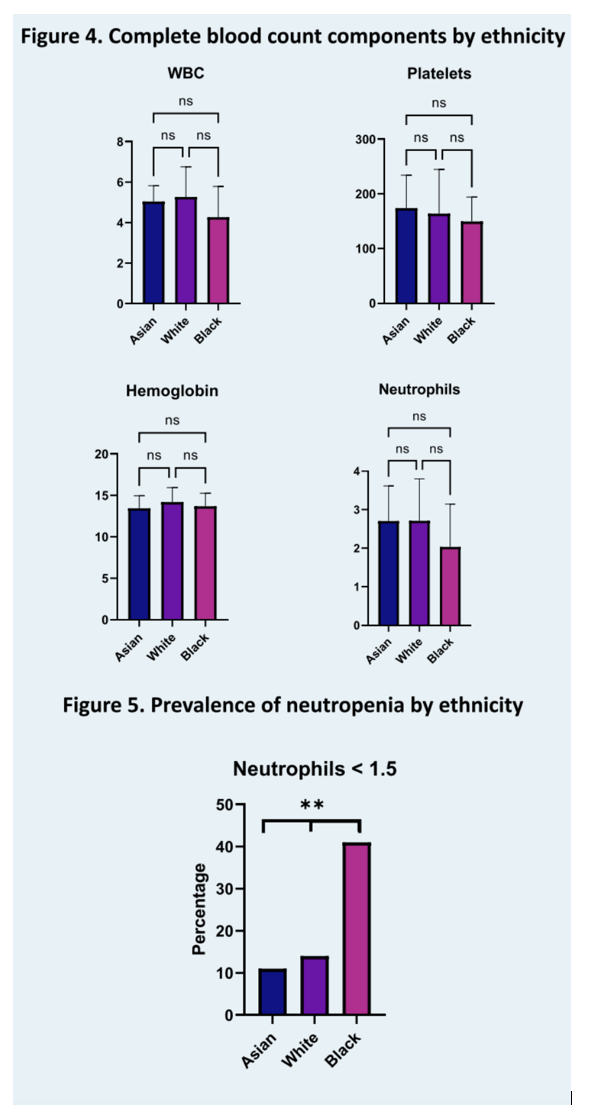
Results: Ninety-eight patients, 47% Asian, 35% White and 17% Black were included in this analysis with an average age at presentation of 43.2 years (standard deviation (SD) 11.5). Overall, 14% were HBe antigen positive. Histological analysis, including mean Ishak fibrosis stage of 3.3 (SD 1.5) and histological activity index of 9.5 (SD 2.9), as well as transient elastography of 12.8 kPa (SD 13.5) were comparable across all groups (respectively p=0.14, p=0.5 and p=0.85). Mean HDV RNA was 5.17 log10 International Units (IU)/mL, 49% of patients had suppressed HBV DNA (<20 IU/mL) and mean platelet count was 166 K/uL (SD 66), without difference between all groups (respectively p=0.98, p=0.9 and 0.4). However, both ALT and AST were significantly higher in Black than in Asian and White patients (ALT respectively 169 U/L, 86 U/L and 87 U/L (p=0.04) and AST 118 U/L, 67 U/L and 58 U/L (p=0.009)). Furthermore, mean absolute neutrophil count (ANC) was 2.7 K/uL in both Asian and White patients as opposed to 2.0 K/uL in Black patients (p=0.0495) with 41% of Black patients presenting with an ANC inferior to 1.5, the minimum recommended baseline value for treatment initiation on the pegylated interferon alfa package insert.
Conclusion: For a similar stage of liver disease at presentation, Black patients have higher biochemical inflammatory markers and lower neutrophil counts, a significant barrier to interferon access, which remains the only recommended therapy for HDV. Ethnic variations in baseline characteristics need to be recognized to ensure equitable access to clinical trials of novel therapies.


|
| |
|
 |
 |
|
|