 |
 |
 |
| |
Largest Case Series of SOF + GLE/PIB + RBV for Deep HCV Salvage: 100% SVR12
|
| |
| |
AASLD, The Liver Meeting, November 12-15, 2021
Mark Mascolini
An anti-HCV salvage regimen of sofosbuvir (SOF) plus glecaprevir (GLE)/pibrentasvir (PIB) and ribavirin (RBV) helped 12 of 12 people with multiple direct-acting antiviral (DAA) failures to attain sustained virologic response 12 weeks after treatment stopped (SVR12) in a US retrospective analysis [1]. No one had to stop the rescue regimen early or had an adverse event requiring hospital admission.
AASLD and EASL guidelines call for 16 to 24 weeks of SOF+GLE/PIB+RBV after failure of another salvage combination, SOF plus velpatasvir (VEL) and voxilaprevir (VOX). But that advice rests on scattered case reports. Researchers from Kaiser Permanente (KP) Northern California and the University of California, San Francisco (UCSF) searched their records to assemble a larger case series of people who used these DAAs.
The analysis involved all adults at KP and UCSF in whom SOF/VEL/VOX salvage failed and the next regimen was SOF+GLE/PIB+RBV. The 12 people identified had a median age of 66 (interquartile range 54 to 67) at treatment. Ten were men, 10 white, 1 black, and 1 Native American. Two people identified Hispanic ethnicity.
Two DAA regimens had failed in 8 people, three regimens had failed in 2, and four had failed in 1. Seven people had hypertension, 5 had diabetes, and median body mass index stood at 28 kg/m2 (in the overweight range). One person had a liver transplant and 1 a kidney transplant.
Eight people had HCV genotype 1a, while 4 had genotype 3. Fibrosis stage was F0 in 2, F1 in 1, F2 in 3, and F4 in 6. Among 6 people with cirrhosis, 3 had Child-Turcotte-Pugh class A5 and 3 had class A6. Three people had esophageal varices and 3 had hepatocellular carcinoma.
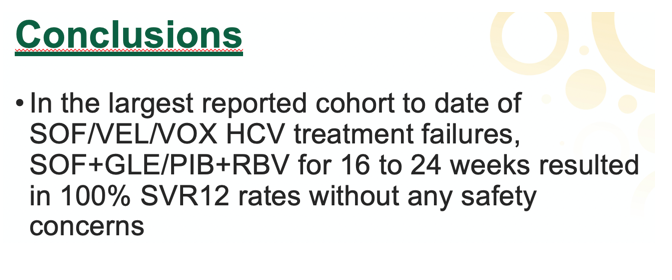
Treatment lasted 16 weeks in 7 people and 24 weeks in 5. Six people took a weight-based RBV dose of 1000 to 1200 mg daily, while 6 took 600 mg daily. Clinicians lowered the RBV dose in 9 people during treatment. Everyone achieved SVR12.
No one stopped treatment early, no one used epoetin alfa, and no one had an adverse reaction requiring hospital admission.
The KP/UCSF team concluded that this largest reported series of people taking SOF+GLE/PIB+RBV after SOF/VEL/VOX failure found that 12 of 12 people attained SVR with no safety concerns.
Reference
1. Saxena V, McKinney J, Chamberland SL, et al. Excellent efficacy and safety of sofosbuvir, glecaprevir, pibrentasvir and ribavirin for retreatment of chronic hepatitis C after sofosbuvir, velpatasvir and voxilaprevir failure. AASLD, The Liver Meeting, November 12-15, 2021. Parallel session 14: Hepatitis C Oral Session.
AASLD: Excellent Efficacy and Safety of Sofosbuvir, Glecaprevir, Pibrentasvir and Ribavirin for Retreatment of Chronic Hepatitis C after Sofosbuvir, Velpatasvir and Voxilaprevir Failure - (11/16/21)
|
| |
|
 |
 |
|
|