 |
 |
 |
| |
IMPACT OF HCV VIRAL PARAMETERS ON PREGNANCY COMPLICATIONS AND RISK OF MOTHER-TO-CHILD TRANSMISSION
|
| |
| |
AASLD Nov 12-15
Tatyana Kushner1,2, Maya Djerboua3, Mia Jaclyn Biondi4, Jordan J Feld4, Norah Terrault5 and Jennifer A. Flemming6, (1)Icahn School of Medicine at Mount Sinai, (2)Division of Liver Diseases, Icahn School of Medicine, Mount Sinai Hospital, (3)Ices, (4)University Health Network, (5)Division of Gastrointestinal and Liver Diseases, University of Southern California, (6)Medicine, Queen's University
Background: To achieve the World Health Organization goals to eliminate HCV by 2030, testing of women in pregnancy is vital and is recommended by multiple societies including most recently the American College of Obstetrics and Gynecology. Pregnancy may represent a unique time for intervention, yet there are limited data on risk of pregnancy complications, including whether a specific viral load cutoff can predict mother-to-child transmission (MTCT) of HCV.
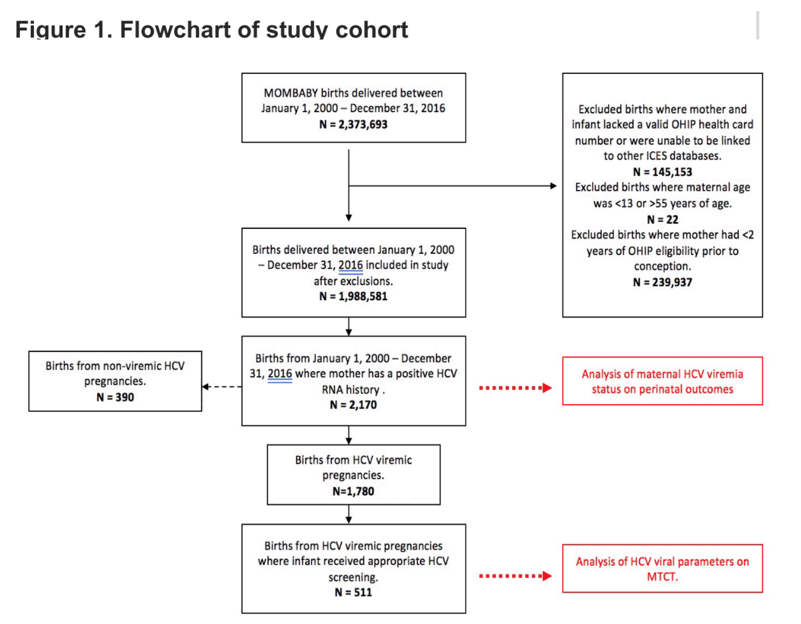
Methods: Retrospective cohort study, using routinely collected Ontario healthcare data from the ICES administrative data repository, of all pregnant women and infants born to women with a history of positive HCV RNA from 2000 to 2014, with follow-up to 2016. Women were stratified as HCV RNA positive or negative during pregnancy using linkage to Public Health Ontario data. Rates and predictors of adverse pregnancy outcomes (stratified by HCV RNA status during pregnancy) were evaluated with adjusted exact odds ratios (eOR). In infants screened for MTCT (either by HCV antibody or RNA at 12 months), sequential HCV viral load cutoffs were evaluated to determine the association with MTCT.
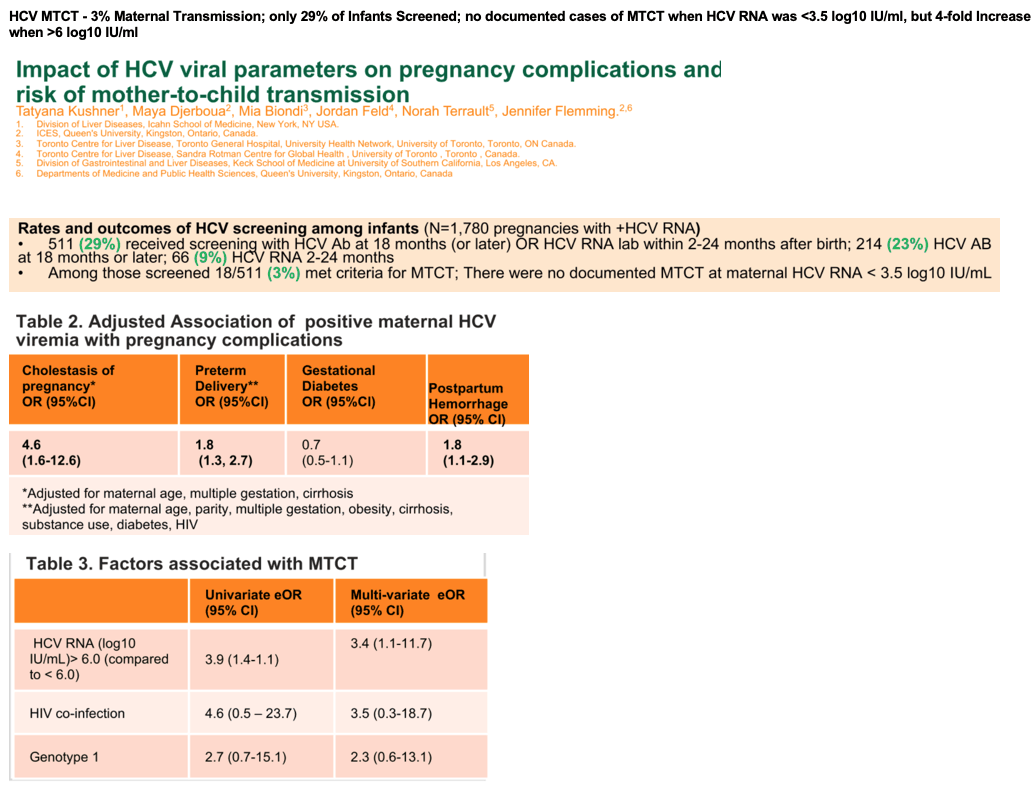
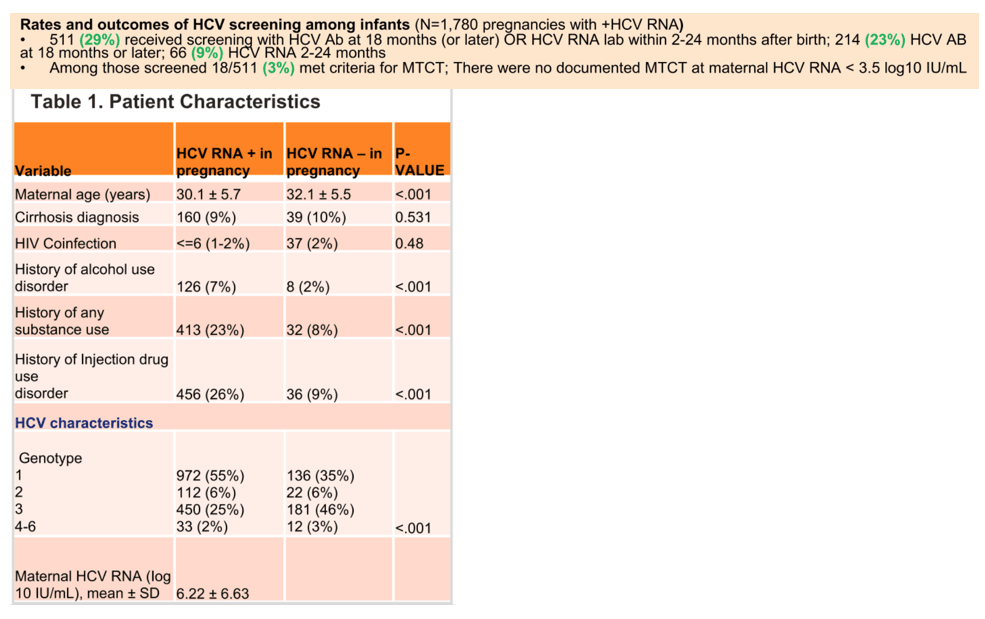
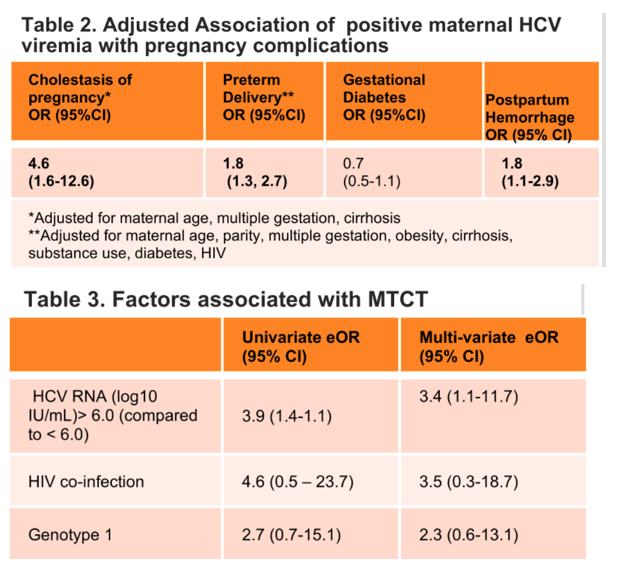
Results: We identified 1,482 pregnancies in women who were HCV RNA+ prior to pregnancy (during pregnancy: n=1,239 RNA+; n=243 RNA-). Women were predominantly genotype (GT) 1 (50%), 69% with HCV RNA available were <6 log10 IU/mL; 2% HIV coinfected; 19% with history of substance use disorder and 18% with documented history of injection drug use. In multivariable models adjusted for maternal age, cirrhosis, multiple gestations, HIV, and substance use, HCV RNA+ pregnancies showed increased odds of preterm delivery (eOR 1.61, p=0.04), postpartum hemorrhage (eOR 2.04, p=0.03), large for gestational age infants (eOR 2.00, p=0.04), and intrahepatic cholestasis of pregnancy (ICP; eOR 3.82, p=0.06). With regards to MTCT, only 385/1,239 (26%) infants were screened by HCV antibody and/or RNA at or after 12 months, with 20/385 (5.2%) having confirmed MTCT. There were no cases of MTCT when maternal HCV RNA was <3.5 log10 IU/mL whereas MTCT occurred in 12 of 112 (11%) women with HCV RNA>6 log/IU/mL (Figure 1). Women with ICP and HCV genotype 1 were more likely to have MTCT (p<0.05). Maternal HCV RNA >6.0 log10 IU/mL adjusted for HIV co-infection and GT was associated with an increased odds of MTCT (eOR 3.89, 95% CI 1.29-13.09, p=0.013).
Conclusion: In a large North American cohort with linked maternal-infant data, women with HCV and active viremia during pregnancy had increased adverse peri-partum outcomes compared to women who were HCV RNA negative. HCV RNA level of >6 log10 IU/mL was associated with an almost 4-fold higher risk of MTCT. Achieving RNA- status prior to pregnancy may reduce the risk of peri-partum complications. Achievement of HCV
|
| |
|
 |
 |
|
|