 |
 |
 |
| |
DURABLE EFFICACY OF DTG+3TC IN GEMINI-1&2: YEAR 3 SUBGROUP ANALYSES
|
| |
| |
CROI 2021 March 6 Reported by Jules Levin
Chloe Orkin1, Norma Porteiro2, Mezgebe Berhe3, Robin H. Dretler4, Federico Pulido5, Shu-Hsing Cheng6, Cristiana Oprea7, Margarate A. Johnson8, Svetlana Kizhlo9, Jörg Sievers10, Choy Man11, Rimgaile Urbaityte12, Mark Underwood11, Brian Wynne11, Jean A. Van Wyk10
1Queen Mary University of London, London, UK, 2Fundacion IDEAA, Buenos Aires, Argentina, 3Texas Infectious Diseases Consultants, Dallas, TX, USA, 4Infectious Disease Specialists of Atlanta, Decatur, GA, USA, 5Hospital Universitario 12 de Octubre, Madrid, Spain, 6Taoyuan General Hospital, Taoyuan, Taiwan, 7Victor Babes Clinical Hospital for Infectious and Tropical Diseases, Bucharest, Romania, 8Royal Free Hospital, London, UK, 9Centre for Prevention and Control of AIDS and Infectious Diseases, St. Petersburg, Russian Federation, 10ViiV Healthcare, Brentford, UK, 11ViiV Healthcare, Research Triangle Park, NC, USA, 12GlaxoSmithKline, Uxbridge, UK
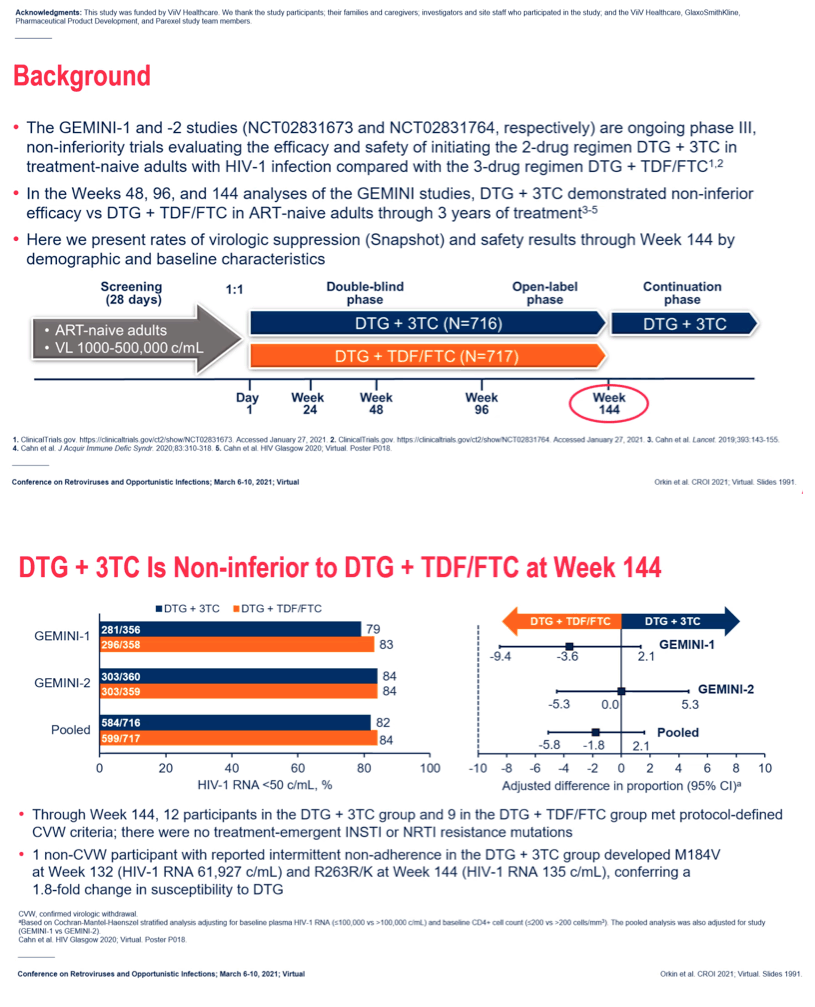
Background: In the GEMINI-1 & GEMINI-2 studies (ClinicalTrials.gov: NCT02831673 & NCT02831764), dolutegravir + lamivudine (DTG+3TC) was non-inferior to the 3-drug regimen of DTG + tenofovir/emtricitabine (TDF/FTC) in achieving plasma HIV-1 RNA <50 c/mL in treatment-naive adults at Weeks 48, 96 and 144.
Methods: GEMINI-1&2 are identical, global, double-blind, multicenter Phase III studies. Participants with screening HIV-1 RNA ≤500,000 c/mL and no major viral resistance mutations to NRTIs, NNRTIs or PIs were randomised to once-daily DTG+3TC or DTG+TDF/FTC, stratified by plasma HIV-1 RNA and CD4+ cell count. The primary endpoint was the proportion of participants with plasma HIV-1
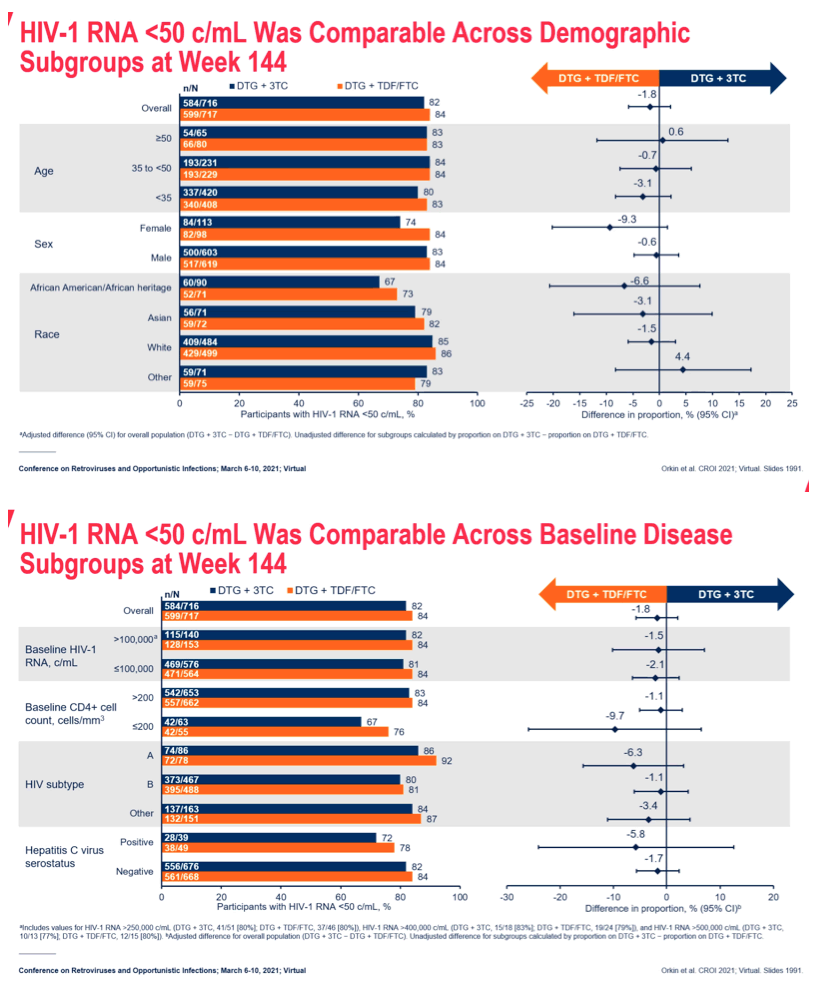
RNA <50 c/mL at Week 48 (Snapshot algorithm). We present a secondary endpoint analysis of efficacy at Week 144 by baseline disease and demographic characteristics. For the overall population, estimates and confidence intervals were based on a stratified analysis using Cochran-Mantel-Haenszel weights.
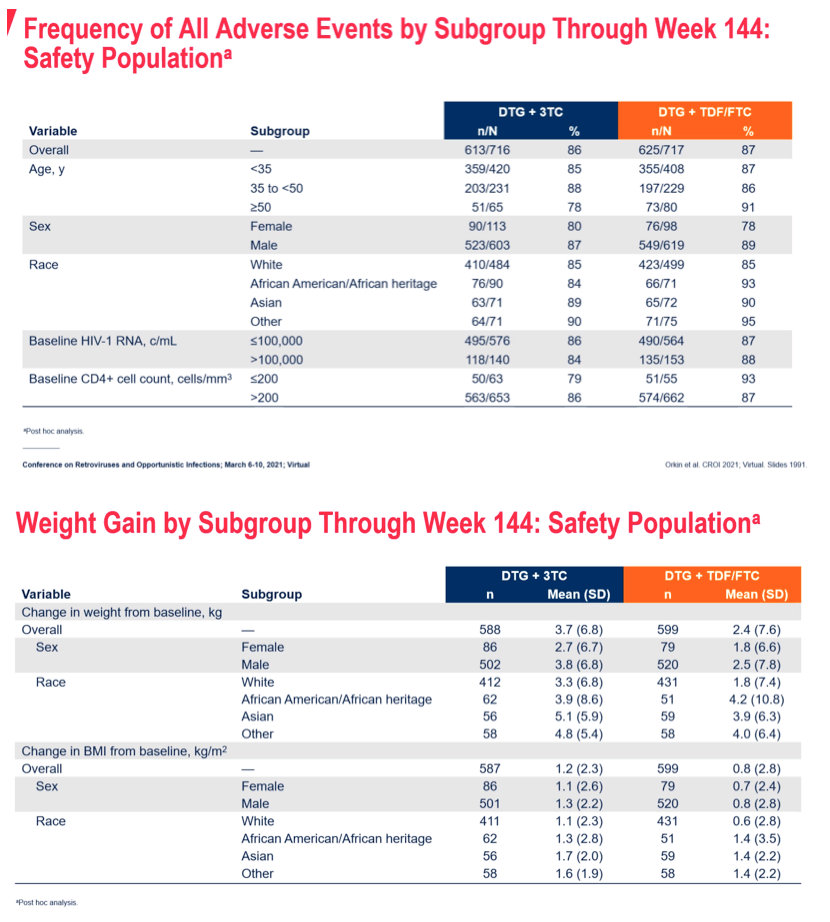
Results: 714 and 719 adults were randomised and treated in GEMINI-1&2, respectively. Using a 10% non-inferiority margin, DTG+3TC was non-inferior to DTG+TDF/FTC at Week 144 in both GEMINI-1&2 and in the pooled analysis. Response rates across baseline HIV-1 RNA subgroups were high and similar in both arms in the pooled analysis, including in participants with baseline HIV-1 RNA >100,000 c/mL (Table 1). Results were also generally consistent regardless of age, sex or race. While response rates remained lower in DTG+3TC compared to DTG+TDF/FTC participants with CD4+ ≤200 cells/mm3, differences were smaller than at Weeks 48 and 96; most reasons for non-response were unrelated to virologic efficacy or treatment regimen. Across both studies, 12 participants on DTG+3TC and 9 on DTG+TDF/FTC met confirmed virologic withdrawal (CVW) criteria through Week 144; none had treatment-emergent InSTI or
NRTI resistance mutations. One non-CVW DTG+3TC participant with reported non-adherence developed M184V at Week 132 and added R263R/K at Week 144, conferring a 1.8-fold change in DTG susceptibility.
Conclusion: In GEMINI-1&2, DTG+3TC was non-inferior to DTG+TDF/FTC in treatment-naive adults at Week 144, demonstrating durable efficacy. The subgroup efficacy results at Week 144 were generally consistent with overall study results and further demonstrate that DTG+3TC is an effective initial treatment for HIV-infected patients across a spectrum of disease characteristics and patient populations.

|
| |
|
 |
 |
|
|