 |
 |
 |
| |
MODEL-INFORMED DOSE SELECTION FOR
ISLATRAVIR/MK-8507 ORAL ONCE-WEEKLY PHASE 2B STUDY
|
| |
| |
Bhargava Kandala1, Craig Fancourt1, Hari Krishna Ananthula1, Youfang Cao1, Pavan Vaddady1, Ernest Asante-Appiah1, Tracy L. Diamond1, Elizabeth G. Rhee1, Randolph P. Matthews1, Wendy Ankrom1, Ryan Vargo1
1Merck & Co, Inc, Kenilworth, NJ, USA
Background:
The novel 2-drug, once weekly (QW) oral combination of Islatravir (ISL) and MK-8507 is in development for the treatment of HIV-1, with the potential to decrease pill burden and dosing frequency. ISL is a first in class NRTTI that is being developed for treatment and prevention of HIV-1. Single doses of MK-8507, a novel NNRTI, achieved robust viral load declines for at least a week post-dose in treatment-naïve people living with HIV (PLWH). Dose selection was determined via modeling and simulation for an ISL+MK-8507 dose ranging Phase 2b study. [NCT04564547]
Methods:
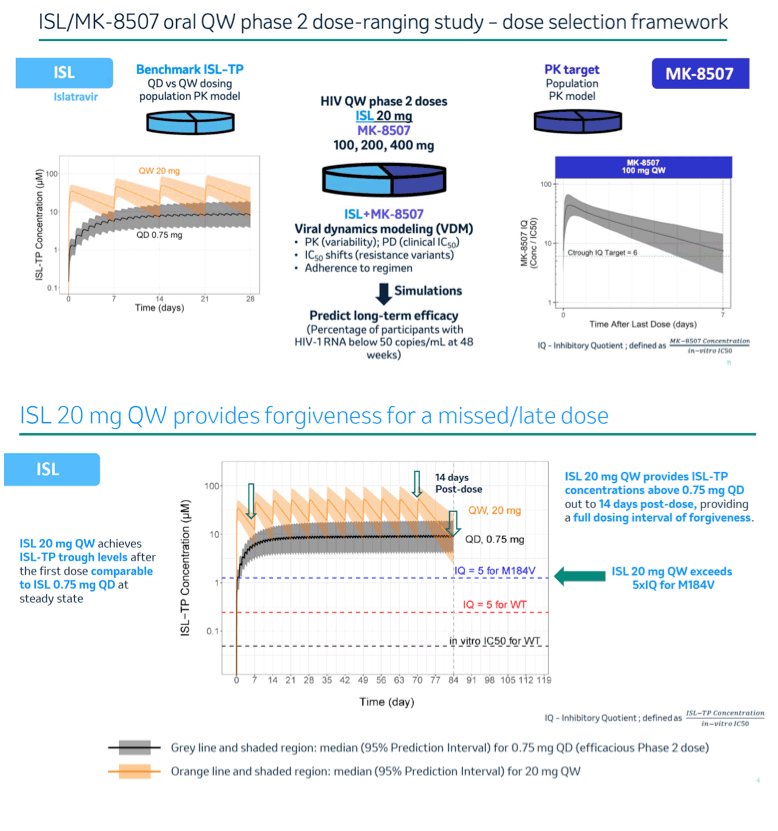
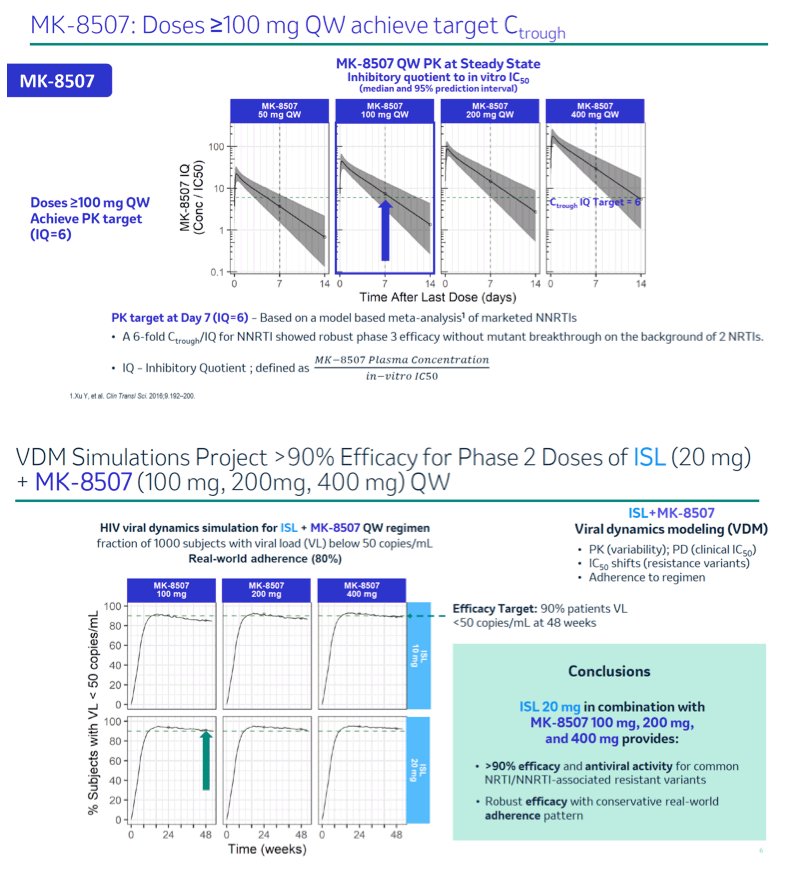
Concentrations of ISL-triphosphate (ISL-TP), the intracellular active moiety, following QW administration of ISL were predicted using a population pharmacokinetic (PK) model. MK-8507 concentrations were also predicted using a population PK model. A Viral Dynamics Model (VDM) for ISL and MK-8507 was used to predict efficacy for a range of QW doses of ISL (5 - 30 mg) and MK-8507 (50 - 400 mg). The VDM combines PK (drug exposures and the associated population variability), pharmacodynamic inhibitory effect (clinical IC50 of ISL and MK-8507 estimated from treatment-naïve monotherapy studies; IC50 fold reduction due to resistance-associated variants) and viral dynamics to predict trial outcome as measured by percent efficacy (% of participants with HIV-1 RNA below 50 copies/mL at 48 weeks). A real-world adherence model developed based on a claims database of PLWH receiving Abacavir/Dolutegravir/ Lamivudine QD was applied.
Results:
A single dose of ISL 20 mg QW achieves ISL-TP trough concentrations comparable to steady state trough levels of ISL-TP for a dose of 0.75 mg QD, a dose shown to provide coverage for wild-type and common NRTI resistance associated variants including M184V. VDM simulations demonstrated that an oral 2-drug QW regimen containing ISL 20 mg in combination with MK-8507 100 mg, 200 mg or 400 mg doses provides 1) at least 90% efficacy and antiviral activity against the most common NRTI and NNRTI resistance-associated variants and 2) robust viral load suppression and efficacy in the event of a late or missed dose and real-world adherence patterns.
Conclusion:
This analysis supports selection of ISL 20 mg in combination with MK-8507 100 mg, 200 mg or 400 mg for further development as a QW regimen.
|
| |
|
 |
 |
|
|