 |
 |
 |
| |
SELF-TESTING FOR HCV: MULTICOUNTRY EVIDENCE ON USABILITY AND ACCEPTABILITY
|
| |
| |
CROI 2021 March 6-10 Reported by Jules Levin
Elena Ivanova Reipold1, Thi Thuy Van Nguyen2, Gamal Shiha3, Ketevan Stvilia4, Aliza Monroe-Wise5, Cheng Wang6, Muhammad Jamil7, Cheryl C. Johnson7, Philippa Easterbrook7
1Foundation for Innovative New Diagnostics, Geneva, Switzerland, 2World Health Organization, Vietnam Country Office, Hanoi, Viet Nam, 3Mansoura University, Mansoura, Egypt, 4National Center for Disease Control, Tbilisi, Georgia, 5University of Washington, Seattle, WA, USA, 6Guangxi Medical University, Nanning, China, 7World Health Organization, Geneva, Switzerland
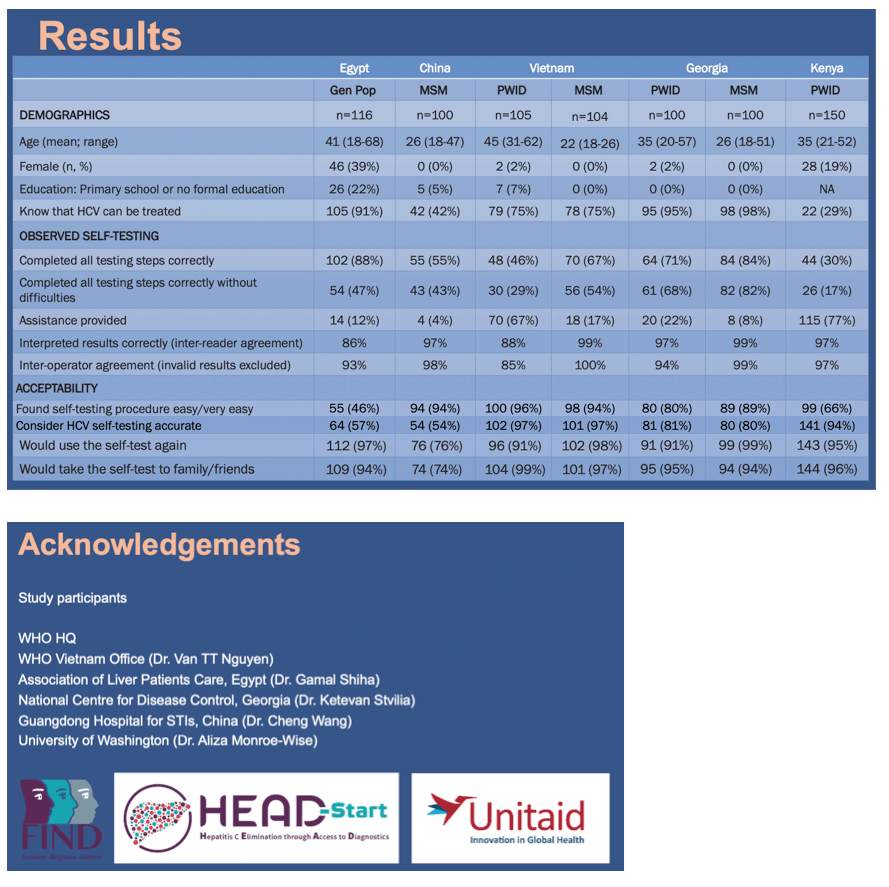
Background: Globally, ≤ 20% of all persons with hepatitis C (HCV) infection have been tested and only one quarter of diagnosed persons have been treated. Self-testing for HCV antibodies (HCVST) may be an additional strategy to expand access to HCV testing and support elimination efforts. We undertook studies to assess usability and acceptability of HCVST in general population as well as key populations: men who have sex with men (MSM) and people who inject drugs (PWID).
Methods: Observational studies were conducted in five countries: Egypt (general population); China (MSM); Kenya (PWID);Vietnam and Georgia (PWID and MSM). Oral fluid OraQuick® HCV Rapid Antibody Test with Instructions for Use (IFU) adapted for ST was used as a prototype HCVST kit. Participants were provided written and pictorial IFU and then conducted ST in a private room with a trained observer. In Egypt, in addition to IFU, study personnel provided a one-to-one demonstration on how to use the test. Usability was evaluated through observer assessment of errors and difficulties during ST using a standardized checklist; and acceptability using a semi-structured questionnaire. HCVST results were read and interpreted by participants and then re-read by the observer. All participants were re-tested with a professional use OraQuick® HCV Test performed by a trained provider.
Results: A total of 775 participants were enrolled across five studies. Participants completing all testing steps without any mistakes was greatest in Egypt and Georgia (>70%), and lowest in PWID from Kenya (30%) and Vietnam (46%). The most common error was incorrect sample collection. Inter-reader agreement ranged from 86% to 99%, and inter-operator agreement from 85% to 99%. The majority of PWID from Vietnam and Kenya required assistance in performing HCVST. The proportion of participants who found the kit very easy or easy to conduct ranged from 55% in Egypt and 66% in Kenya, to more than 80% in other countries. Acceptability was high with > 90% of participants in four countries willing to use HCVST again and who would recommend it to family and friends.
Conclusion: These are the first studies globally to demonstrate high usability and acceptability of HCVST in both general and key populations. While most users self-tested with ease, assisted self-testing models are needed for some populations such as PWID. HCVST is an important strategy for further consideration as it may be a promising tool for increasing coverage and achieving elimination goals.


|
| |
|
 |
 |
|
|