 |
 |
 |
| |
HEPATITIS C CASCADE OF CARE IN HIV/HCV-COINFECTED PERSONS IN EUROPE IN THE DAA ERA
|
| |
| |
CROI 2021 March 6-10 Reported by Jules Levin
Olga Fursa1, Amanda Mocroft2, Jeffrey V. Lazarus3, Sarah Amele2, Jens D. Lundgren1, Raimonda Matulionyte4, Line D. Rasmussen5, Jürgen K. Rockstroh6, Milosz Parczewski7, David Jilich8, Santiago Moreno9, Anna Vassilenko10, Karine Lacombe11, Lars Peters1, for the EuroSIDA Study Group
1Centre of Excellence for Health, Immunity and Infections, Copenhagen, Denmark, 2Centre for Clinical Research, Epidemiology, Modelling and Evaluation, Institute for Global Health, University College London, London, UK, 3Barcelona Institute for Global Health, Barcelona, Spain, 4Vilnius University, Vilnius, Lithuania, 5Odense University Hospital, Odense, Denmark, 6Bonn University Hospital, Bonn, Germany, 7Pomeranian Medical University, Szczecin, Poland, 8Charles University in Prague and Na Bulovce Hospital, Prague, Czech Republic, 9Hospital Universitario Ramón y Cajal, Madrid, Spain, 10Belarusian State Medical University, Minsk, Belarus, 11Institut Pierre Louis d'Epidémiologie et de Santé Publique, Paris, France
Background: The WHO global hepatitis C (HCV) elimination targets include diagnosing 90% and treating 80% of HCV infected individuals by 2030. We described the HCV cascade of care (CoC) for people living with HIV (PLHIV) across Europe to assess progress towards reaching these two targets.
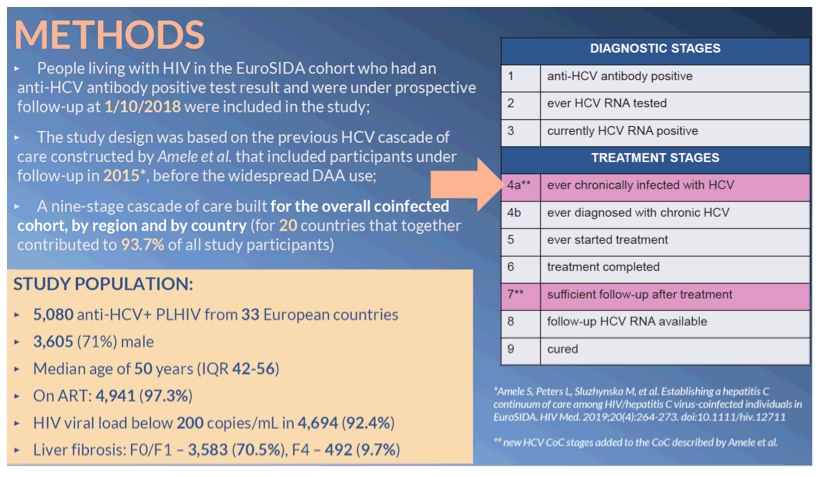
Methods: HIV/HCV-coinfected participants in the EuroSIDA cohort under prospective follow-up at 1 October 2018 were described using a CoC with 3 diagnostic stages - anti-HCV positive, ever HCV RNA tested, currently HCV RNA positive; and 7 treatment stages - ever chronically infected (multiple imputation for persons with missing HCV RNA test data), ever diagnosed with chronic HCV, ever treated, completed treatment, sufficient follow-up available, follow-up HCV RNA available, cured. CoC were compared across five European regions and 20 countries enrolling >50 persons.
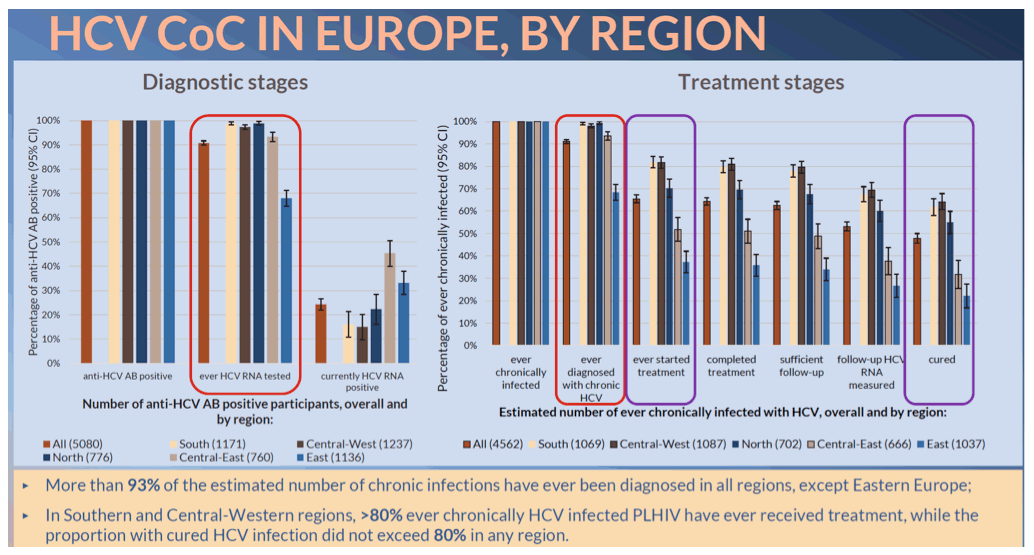
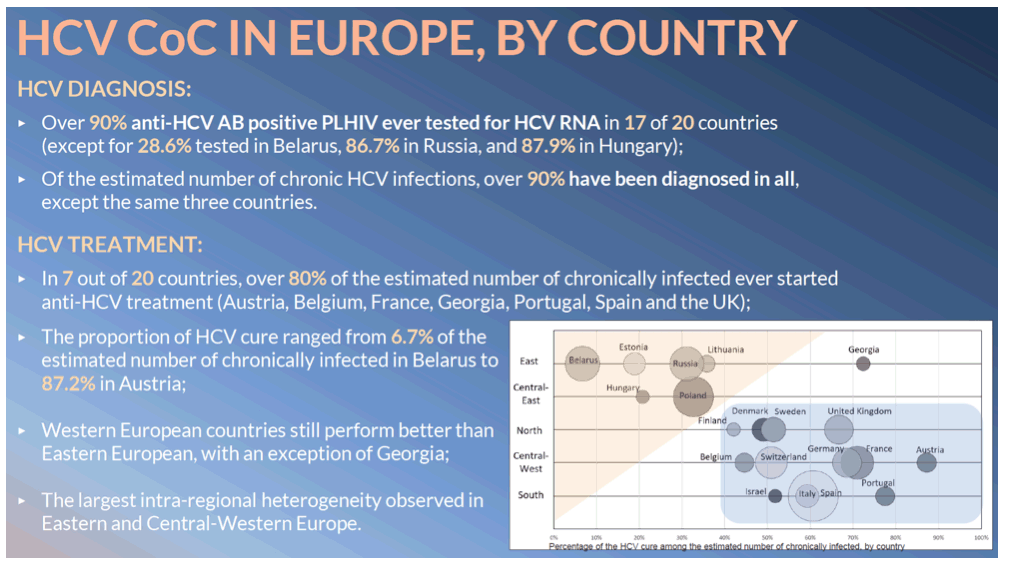
Results: Of 5,080 anti-HCV positive persons, 4,609 (90.7%, 95% confidence interval (CI) 89.9-91.6) were ever HCV RNA tested (Figure 1A) and 24.3% of individuals (95% CI 21.9-26.7) were currently HCV RNA positive, with higher prevalence in Central-East and East (45.4% and 33.2%, respectively). Among all participating countries the proportion of currently HCV RNA positive was the highest in Estonia (62% of 160 anti-HCV positive) and lowest in Austria (4.8% of 124). An estimated 4,562 (89.8%, 95% CI 88.9-90.7) anti-HCV positive individuals have been ever chronically infected, of which 4,155 persons (90.1%, 95% CI 89.2-91.0) have been ever diagnosed with chronic HCV (Figure 1B). In Eastern Europe, 68.4% of chronic infections have ever been diagnosed, with >93% in the other four regions. Overall, 2,989 persons have ever been treated (65.5% of the ever chronically infected, 95% CI 63.8-67.2) and 2,186 individuals (47.9% of the ever chronically infected, 95% CI 45.8-50) were cured. Cure proportion ranged from 6.7% of 356 ever chronically infected in Belarus to 87.2% of 109 in Austria.
Conclusion: In all regions except Eastern Europe, >90% of anti-HCV positive PLHIV under follow-up at 1 October 2018 have been tested for HCV RNA. In South and Central-West, >80% ever chronically HCV infected PLHIV have ever received treatment. Overall, the proportion with cured HCV infection did not exceed 80% in any region with significant heterogeneity between countries. Increased access to affordable direct acting antivirals, particularly in Eastern Europe, is required to achieve HCV elimination by 2030 among PLHIV in Europe.

|
| |
|
 |
 |
|
|