 |
 |
 |
| |
NO CHANGE IN INCIDENCE OF RECENTLY ACQUIRED HCV IN HIV+ MSM IN GERMANY (NOCO COHORT)
|
| |
| |
CROI 2021 March 6-10 Reported by Jules Levin
Patrick Ingiliz1, Natasha Martin2, Thomas Lutz3, Knud C. Schewe4, Stefan Mauss5, Stefan Christensen6, Sonia Jain7, Feng He7, Martin Daeumer8, Axel J. Schmidt9, Michael Sabranski4, Axel Baumgarten1, Markus Bickel3, Jürgen K. Rockstroh10, Christoph Boesecke10
1Center for Infectiology, Berlin, Germany, 2Division of Infectious Diseases and Global Public Health, University of California San Diego, San Diego, CA, USA, 3Infektiologikum, Frankfurt, Germany, 4Infectiologisches Centrum, Hamburg, Germany, 5Center for HIV and Hepatogastroenterology, Düsseldorf, Germany, 6Muenster University Hospital, Muenster, Germany, 7University of California San Diego, La Jolla, CA, USA, 8Institute for Genetics and Immunology, Kaiserslautern, Germany, 9London School of Hygiene & Tropical Medicine, London, UK, 10Bonn University Hospital, Bonn, Germany
Background: Directly-acting antiviral agents (DAA) against the hepatitis C virus (HCV) have been available in Germany since February 2014. Men who have sex with men (MSM) have been identified as one subgroup with continuous HCV transmission and as a target for HCV micro-elimination efforts. We assess newly acquired HCV among MSM in Germany.
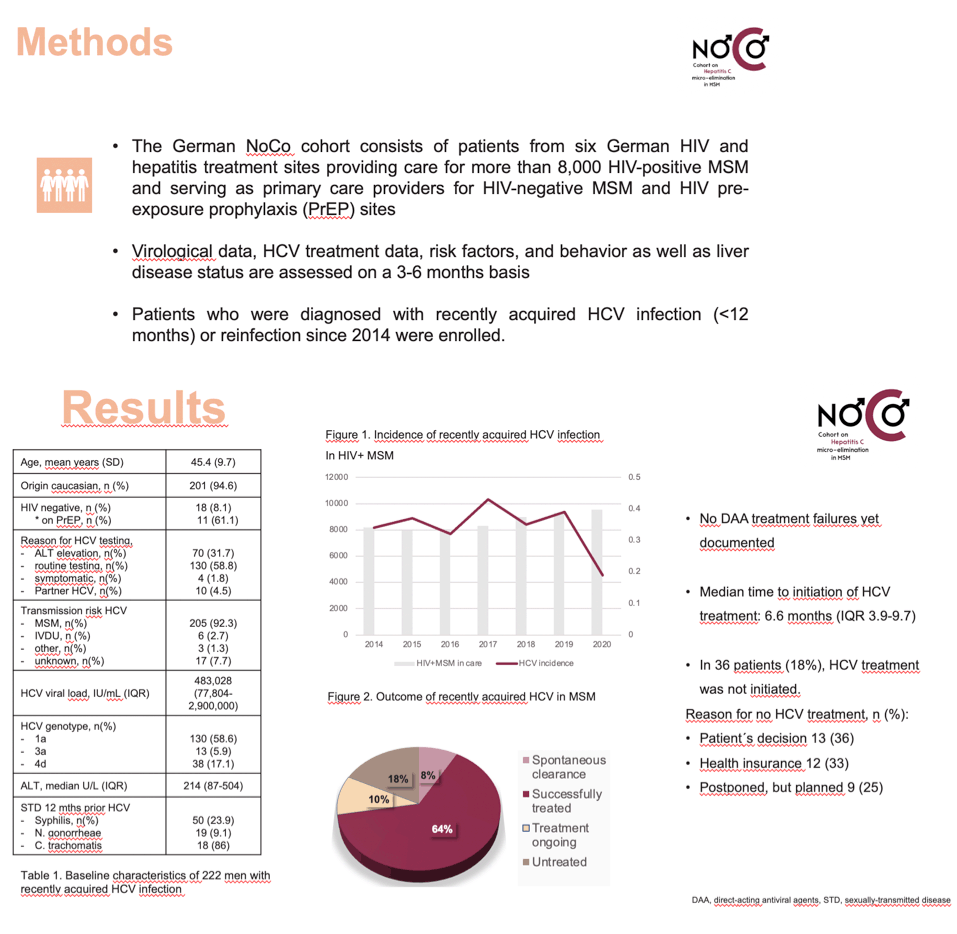
Methods: The German NoCo cohort consists of patients from six German HIV and hepatitis treatment sites providing care for more than 8000 HIV-positive MSM, and serving as primary care providers and HIV pre-exposure prophylaxis (PrEP) sites. Patients who were diagnosed with recently acquired HCV infection since 2014 were enrolled. Virologic data, HIV and HCV treatment data, risk factors and behavior as well as liver disease assessment is acquired regularly.
Results: Between January 2014 and September 2020, 214 MSM with recently acquired HCV infection were included. A majority were Caucasian (94%), and mean age was 45.5 years (standard deviation, SD, 9.64). At HCV diagnosis, median ALT level was 201 U/L (interquartile range, IQR, 86- 509), and median HCV viral load was 483,028 IU/mL (IQR 77,804 - 2,525,000). The most prevalent HCV genotype was 1a (58.9%), followed by genotype 4d (16.4%), and 3a (6.1%). The risk factors for HCV acquisition were as follows: MSM: 92.5%, intravenous drug use: 2.8%, intranasal drug use: 0.9%, other: 0.5%. A subgroup of 17 (7.8%) MSM were not co-infected with HIV, of whom 10 (58.8%) were using PrEP. In 198/214 (92.5%) patients outcome data were available: DAA treatment was documented in 148 patients (74.7%), 16/198 (8.1%) had a spontaneous clearance, and in 34 patients (17.2%) treatment was not started, in most cases (35.3%) due to health insurance constraints. Among those treated, DAAs were initiated a median 6.6 months (IQR 3.4 to 9.6) after diagnosis; all treated patients achieved a sustained virologic response (SVR), or treatment was still ongoing (14%). Between 2014-2019, 26-36 patients were diagnosed with recently acquired HCV annually. In relation to all HIV-positive MSM under care, the incidence was 0.32 - 0.39% per year with no significant change over time.
Conclusion: In this preliminary analysis from the German NoCo cohort, HCV incidence remained stable despite a broad use of DAAs. Delays to HCV treatment initiation and health insurance constraints may fuel ongoing HCV transmission, as well as continuous or even increasing risk behavior.


|
| |
|
 |
 |
|
|