 |
 |
 |
| |
ACCESS TO HCV CARE AMONG HIV/HCV-COINFECTED PEOPLE WHO INJECT DRUGS ACROSS CANADA
|
| |
| |
CROI 2021 March 6-10 Reported by Jules Levin
Charlotte Laniece Delaunay1, Mathieu Maheu-Giroux1, Gayatri Marathe1, Sahar Saeed2, Curtis Cooper3, Sharon Walmsley4, Joseph Cox5, Mark Hull6, Valérie Martel- Laferriere7, Marina B. Klein5
1McGill University, Montreal, Canada, 2Washington University in St Louis, St Louis,
MO, USA, 3University of Ottawa, Ottawa, Canada, 4University of Toronto, Toronto, 5Canada, ResearchInstituteofMcGillUniversityHealthCentre,Montreal,Canada,
6 University of British Columbia, Vancouver, Canada, 7 Centre de Recherchedu CHUM, Montreal, Canada
Background: In North America, 81% of new HCV infections occur among people who inject drugs (PWID), and coinfection with HIV can exacerbate disease severity. Quantifying unmet needs in HCV prevention and treatment among HIV-HCV coinfected PWID is key for developing appropriate interventions to eliminate HCV as a public health threat by 2030. We investigated temporal trends in 1) HCV treatment uptake and efficacy; 2) injection practices; and 3) access to harm reduction programs among HIV-HCV coinfected PWID in Canada from 2003 to 2019.
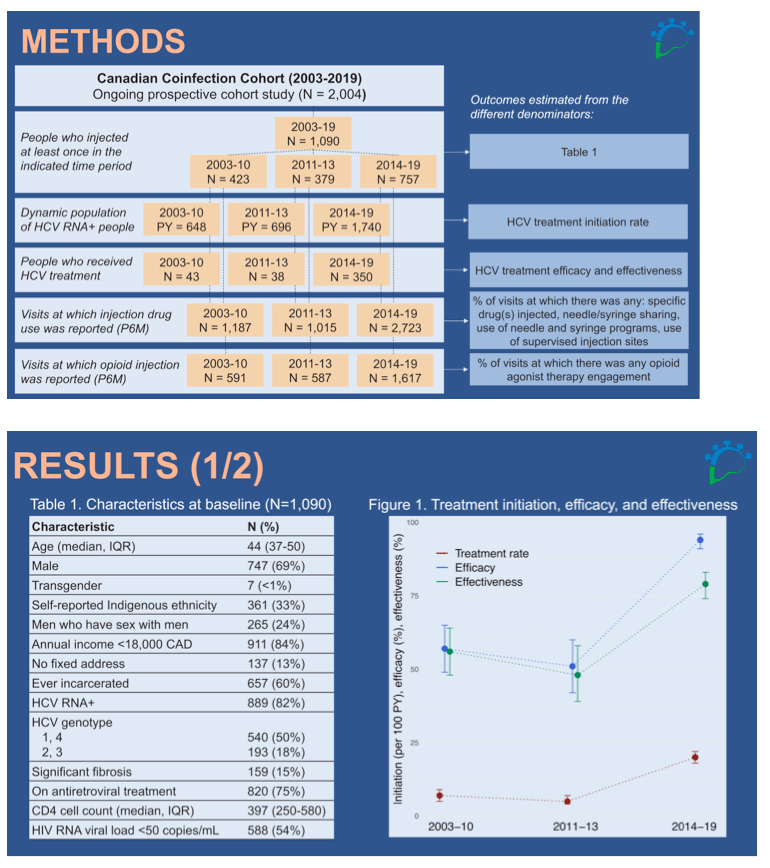
Methods: We used data from the Canadian Coinfection Cohort, a prospective cohort study of 2,004 HIV-HCV coinfected patients. We included 1,090 participants from Quebec, Ontario, Saskatchewan, and British Columbia who reported injecting at least once between 2003 and 2019. Trends were examined using 3 time periods based on HCV treatment guidelines: 2003-2010: interferon/ ribavirin-based; 2011-2013: 1st generation direct-acting antivirals (DAAs); and 2014-2019: 2nd generation DAAs. The harm reduction services assessed include needle and syringe programs (NSP), opioid agonist therapy (OAT), and supervised injection sites (SIS).
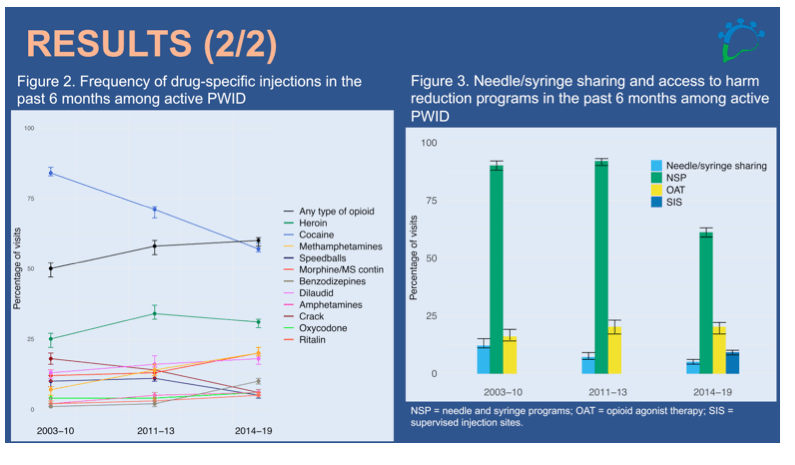
Results: The participants' median age was 44 years; 69% were male; 33% were Indigenous. The overall HCV treatment rate among HCV RNA-positive people increased from 7 per 100 person-years (PY, 95%CI: 5-9) in 2003-2010 to 20 per 100 PY (95%CI: 18-22) in 2014-2019. In the 2nd generation DAA era, treatment efficacy was >90%, compared to 57% in 2003-2010. Cocaine remained the most frequently injected drug among active PWID, but its consumption decreased from 84% (95%CI: 83-86) of visits in 2003-2010 to 57% (95%CI: 56-59) in 2014-2019. Opioid injection increased from 50% (95%CI: 47-52) of visits in 2003-2010 to 60% (95%CI: 58-61) in 2014-2019. Report of needle/syringe sharing declined from 12% (95%CI: 11-15) in 2003-2010 to 5% (95%CI: 4-6) in 2014-2019, yet paradoxically, report of NSP also decreased (Figure). This might reflect a reduction in number of daily injections due to reduced cocaine use. OAT engagement among opioid injectors was also low (≤20%), with no clear temporal trend. SIS data became available in 2014-2019 (reported at 9% of visits (95%CI: 8-10)).
Conclusion: HCV treatment access and outcomes have greatly improved among coinfected PWID. Yet, exposure to injection-related risks continues and is increasingly related to opioid use. There is a need to maximize access to proven harm reduction strategies to prevent HCV re-infection and overdose.

|
| |
|
 |
 |
|
|