 |
 |
 |
| |
INCIDENT DIABETES ASSOCIATED WITH INTEGRASE STRAND TRANSFER INHIBITOR INITIATION
|
| |
| |
CROI 2021 March 6-10 Reported by Jules Levin
Jane O'Halloran1, John Sahrmann1, Margaret Olsen1, William Powderly1 1Washington University in St Louis, St Louis, MO, USA
Background: Integrase strand transfer inhibitors (InSTIs), in particular dolutegravir, have been associated with weight gain in people with HIV (PWH). However, limited data exists on other metabolic outcomes in PWH on this class of ART. We examined the risk of new-onset diabetes mellitus and hyperglycemia in PWH on InSTI-based regimens.
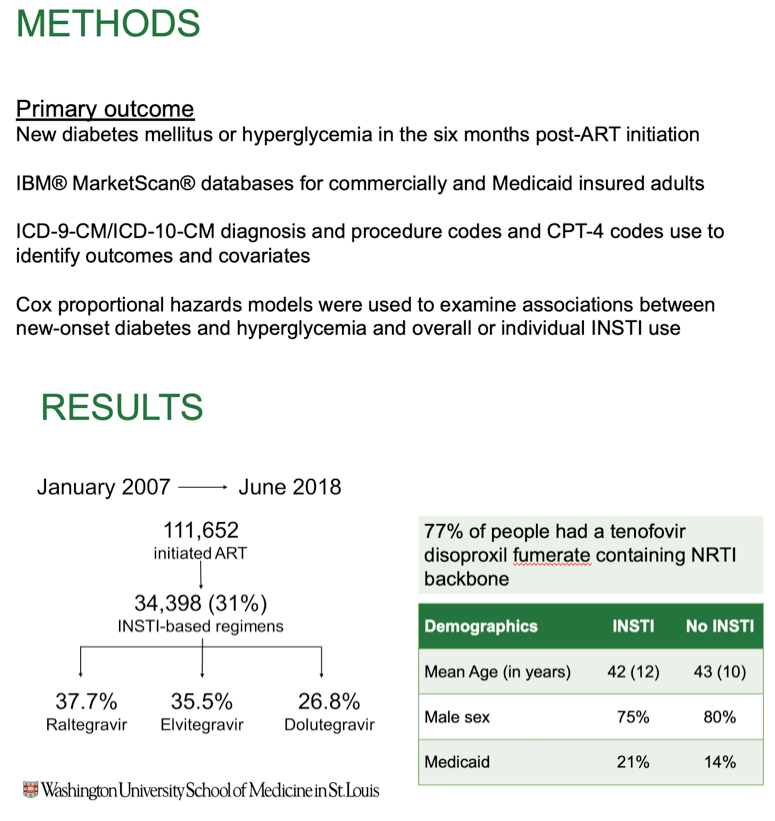
Methods: Data from the IBM® MarketScan® databases for commercially and Medicaid insured adults were used to identify PWH on ART. The date of initiation or switch to InSTI was used as the index date for InSTI users while the date of initiation or 180 days after the first claims (for prevalent users) was used for non-InSTI users. The primary outcome was a composite of new-onset diabetes mellitus or hyperglycemia in the six months post-ART initiation. We identified outcomes and covariates associated with risk of diabetes mellitus and hyperglycemia using ICD-9-CM/ICD-10-CM diagnosis and procedure codes and CPT-4 codes. Cox proportional hazards models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for association between new-onset diabetes and hyperglycemia and overall or individual InSTI use.
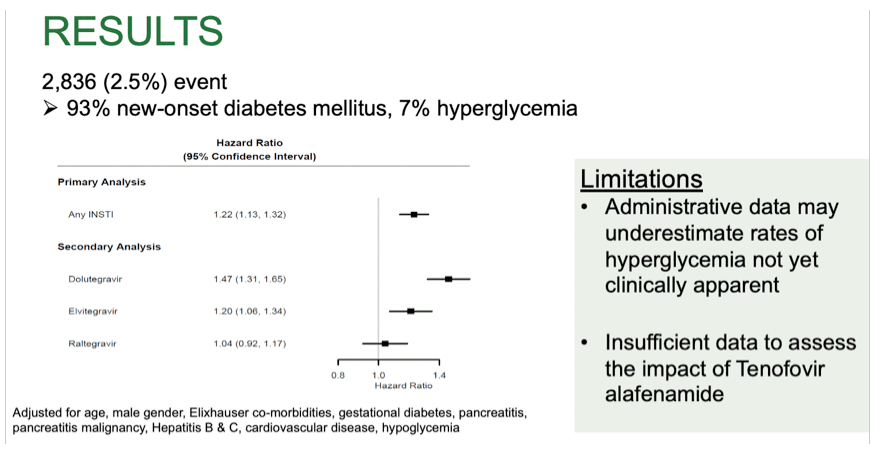
Results: 111, 652 PWH initiated ART between Jan 1, 2007 and June 30, 2018. 34,398 (31%) were treated with InSTI-based regimens (raltegravir 37.7%, elvitegravir 35.5%, dolutegravir 26.8%). Mean age was 42.3 (standard deviation 10.9) years, 78% were male, and 16% were Medicaid insured. The primary outcome occurred in 2,836 PWH (93% new-onset diabetes mellitus, 7% hyperglycemia). PWH on InSTIs were 22% more likely to develop new-onset diabetes mellitus or hyperglycemia (HR 1.22 [95% CI 1.13, 1.32]). When InSTIs were included in the model individually, PWH on dolutegravir were 47% more likely to develop new-onset diabetes mellitus or hyperglycemia (HR 1.47 [95% CI 1.31, 1.66], while those on elvitegravir were 20% more likely (HR 1.20 [95% CI 1.01, 1.34]). No association was observed in those on raltegravir-based therapy (HR 1.04 [95% CI 0.92, 1.17]).
Conclusion: Overall, InSTI use was associated with increased risk of new-onset diabetes mellitus or hyperglycemia in the six months post index. The risk was more than twice as high in those on dolutegravir compared with those on elvitegravir while raltegravir was not associated with this finding. Further research is necessary to understand the mechanism driving these findings.

|
| |
|
 |
 |
|
|