 |
 |
 |
| |
THE ANTIRETOVIRAL PREGNANCY REGISTRY:
30 YEARS OF MONITORING FOR CONGENITAL ANOMALIES
|
| |
| |
CROI 2021 March 6-10 reported by Jules Levin
Jessica D . Albano1, William R. Short2, Angela E. Scheuerle3, Karen Beckerman4, Lynne Mofenson5, Vani Vannappagari6
1Syneos Health, Wilmington, NC, USA, 2University of Pennsylvania, Philadelphia, PA, USA, 3University of Texas Southwestern, Dallas, TX, USA, 4Icahn School of Medicine at Mt Sinai, New York, NY, USA, 5Elizabeth Glaser Pediatric AIDS Foundation, Washington, DC, USA, 6ViiV Healthcare, Research Triangle Park, NC, USA
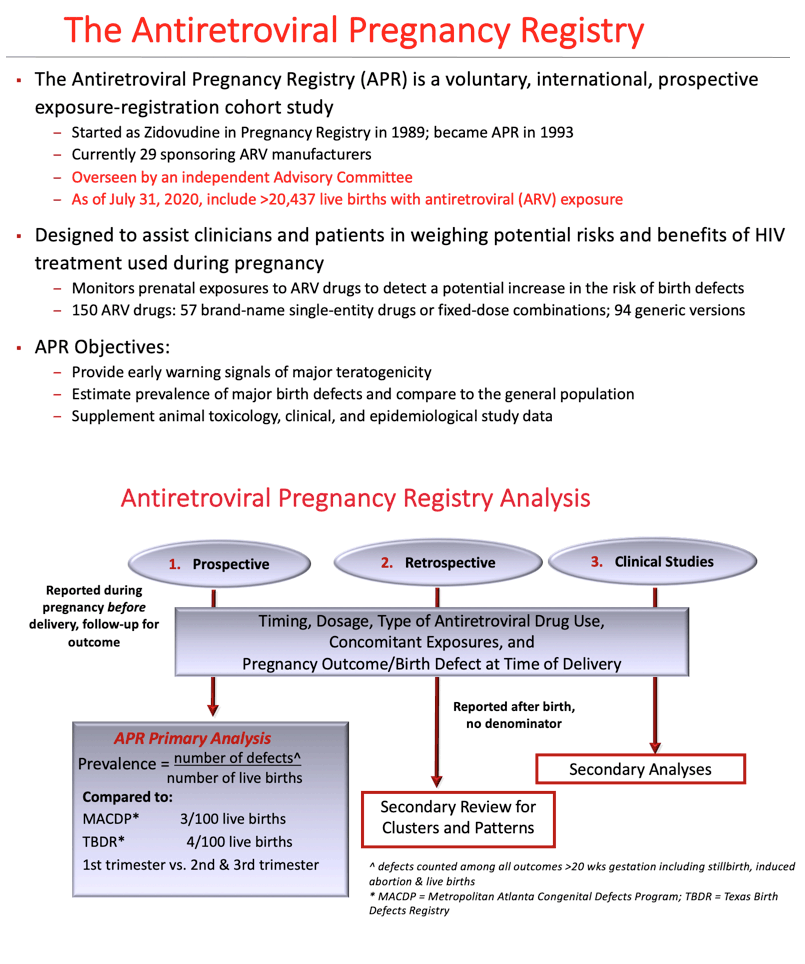
Background: Antiretrovirals (ARVs) effectively reduce perinatal transmission of HIV. The Antiretroviral Pregnancy Registry (APR) began monitoring prenatal ARV use in 1989 to provide an early signal of teratogenicity and to estimate the prevalence of congenital anomalies (CAs) which could be compared to the general population.
Methods: The APR is a prospective exposure-registration cohort study with data from 70 countries. Prevalence of CAs are estimated and compared to internal and external comparison groups. Twenty-two ARVs have sufficient 1st trimester exposures to detect at least a 2-fold increase in risk of CAs overall, of which 11 can detect a 1.5-fold increase.
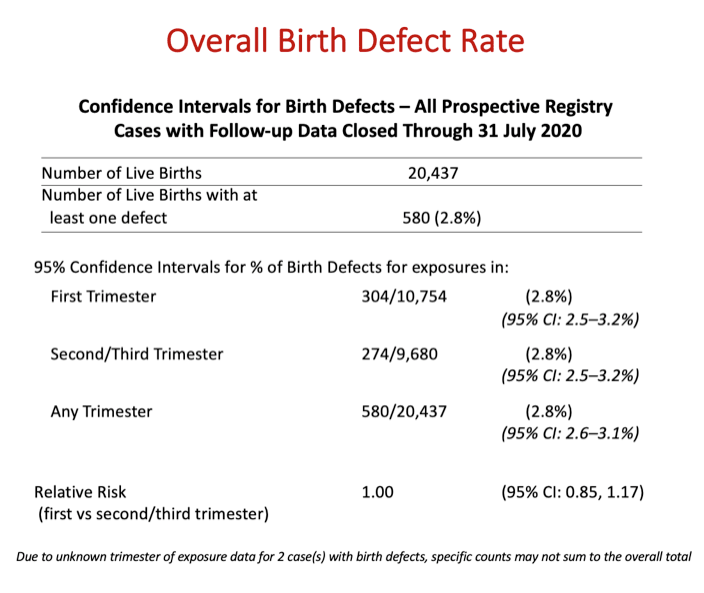
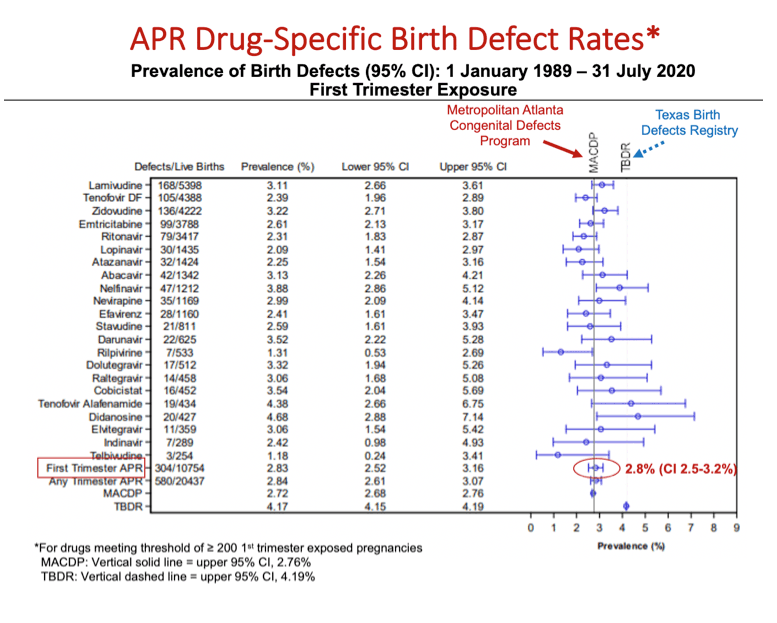
Results: Of the 21,599 evaluable pregnancies enrolled through 31July2020, there were 20,437 live births (LB) with ARV exposure at any time during pregnancy and 580 CAs. Prevalence of CAs was 2.8% (95% CI 2.6-3.1) overall, 2.8% (95% CI 2.5-3.2) among 1st trimester ARV exposures and 2.8% (95% CI 2.5-3.2) among 2nd/3rd trimester ARV exposures to ARVs. Prevalence Ratio comparing 1st vs 2nd/3rd trimester ARV exposures was 1.00 (95% CI 0.85-1.17). Two ARVs, didanosine (DDI) and nelfinavir (NFV) have a modest statistically significant increase in overall prevalence compared to Metropolitan Atlanta Congenital Defects Program (MACDP) but not Texas Birth Defects Registry (TBDR); lower bound of the confidence interval for DDI (2.9%) and NFV (2.9%) is slightly above the higher bound for the comparator MACDP rate. Having reached the threshold of 200 first trimester exposed cases, the prevalence of CAs for tenofovir alafenamide (TAF) is 4.4% (95% CI 2.7-6.8). However, the lower end of the 95% CI is within the upper bound of the 95% CI for both the MACDP and the TBDR.
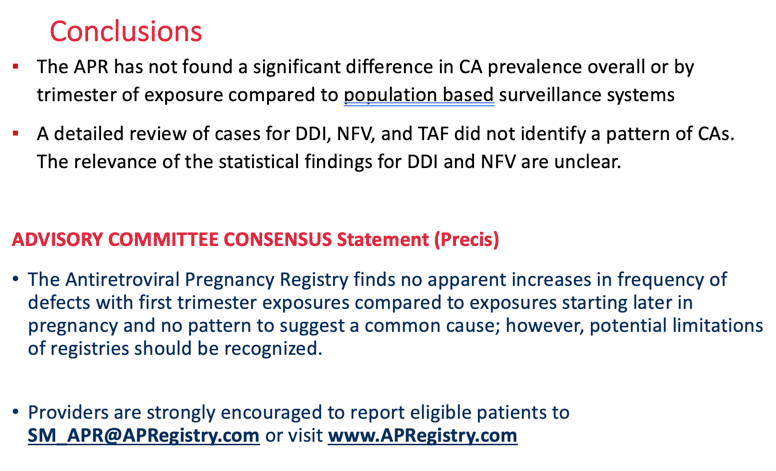
Conclusion: The APR has not found a significant difference in CA prevalence overall or by trimester of exposure compared to two population based surveillance systems: 2.72/100 LB (95% CI 2.68-2.76) MACDP and 4.17/100 LB (95% CI 4.15-4.19) TBDR. A detailed review of cases for DDI, NFV, or TAF did not identify a pattern of CAs. The clinical relevance of the statistical findings for DDI and NFV are unclear. Monitoring will continue. The APR independent Advisory Committee concludes, "The Registry finds no apparent increases in frequency of specific defects with first trimester exposures and no pattern to suggest a common cause; however, potential limitations of registries should be recognized".

|
| |
|
 |
 |
|
|