 |
 |
 |
| |
A SIMPLE AND SAFE APPROACH TO HCV TREATMENT:
FINDINGS FROM THE A5360 (MINMON) TRIAL
|
| |
| |
CROI 2021 March 6-10 Reported by Jules Levin
Sunil S . Solomon, Sandra Wagner-Cardoso, Laura M. Smeaton, Leonard Sowah, Chanelle Wimbish, Gregory Robbins, Irena Brates, Nelson Cheinquer, Anchalee Avihingsanon , Donald Anthony, Benjamin Linas David Wyles , Mark S. Sulkowski, Susanna Naggie
The Johns Hopkins University School of Medicine, Baltimore, MD, USA, Institutonde Pesquisa Clinica Evandro Chagas, Rio de Janeiro, Brazil, Harvard TH Chan School of Public Health, Boston, MA, USA, National Institute of Allergy and Infectiousn Diseases, Bethesda, MD, USA, Social and Scientific Systems,aDLHCompany,Silver Spring,MD, MassachusettsGeneralHospital,Boston,MA,USA, GileadSciences, Inc, Foster City, CA, USA, Thai Red Cross AIDS Research Center, Bangkok, Thailand, Case Western Reserve University, Cleveland, OH, USA, Boston Medical Center, Boston, MA, USA, Duke University Medical Center, Durham, NC, USA, University of Colorado School of Medicine, Aurora, CO, USA
Background: To achieve global hepatitis C virus (HCV) elimination by 2030, 80% of the ∼71 million people with chronic HCV need to be treated, necessitating simplification of treatment delivery and associated laboratory monitoring without compromising efficacy or safety. The COVID-19 pandemic has further highlighted the need for innovative models that minimize face-to- face contact.
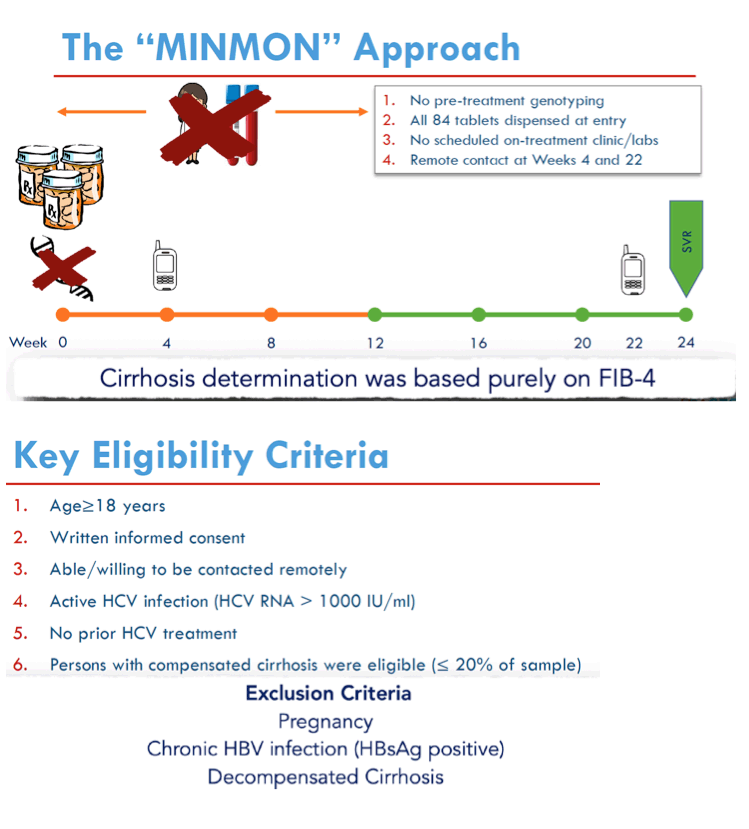
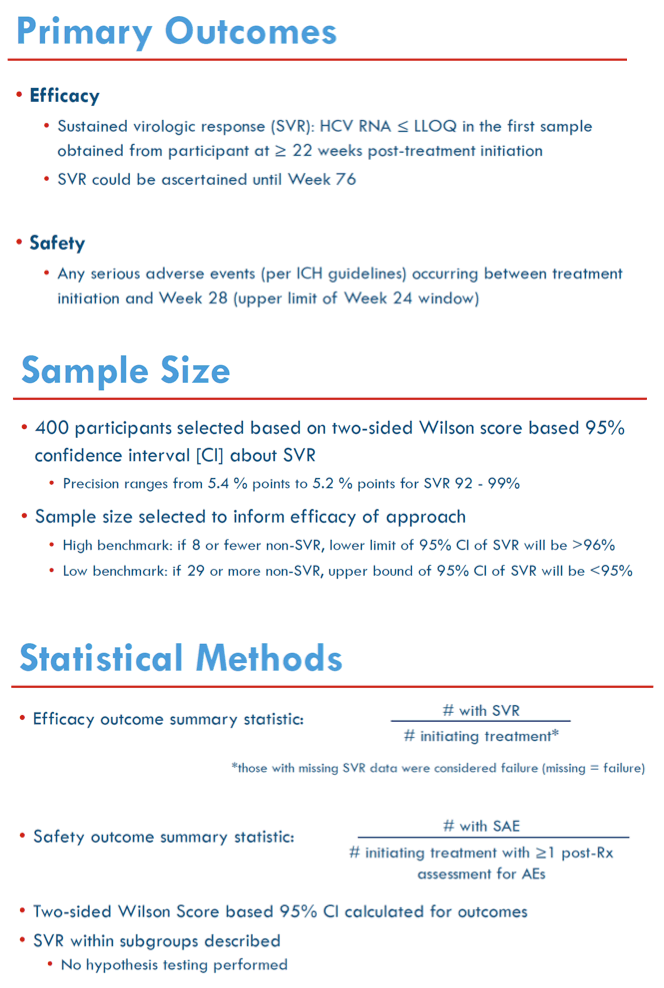
Methods: ACTG A5360 is a single-arm, open-label trial to evaluate safety and efficacy of a minimal monitoring (MINMON) approach to HCV therapy in treatment-naïve persons with no evidence of decompensated cirrhosis. All participants received a single-tablet, fixed-dose regimen of sofosbuvir/velpatasvir for 12 weeks. MINMON included: (1) no genotyping; (2) all tablets dispensed at entry; (3) no on-treatment visits/labs; and (4) two remote contacts at Weeks 4 (adherence assessment) and 22 (scheduling sustained virology response [SVR] visit). Unplanned visits for participant concerns (related/unrelated to an adverse event [AE]) were allowed. SVR is defined as HCV RNA < lower limit of quantification at least 22 weeks after treatment initiation (missing HCV RNA = failure). 95% confidence intervals (CI) for SVR used Wilson's Score.
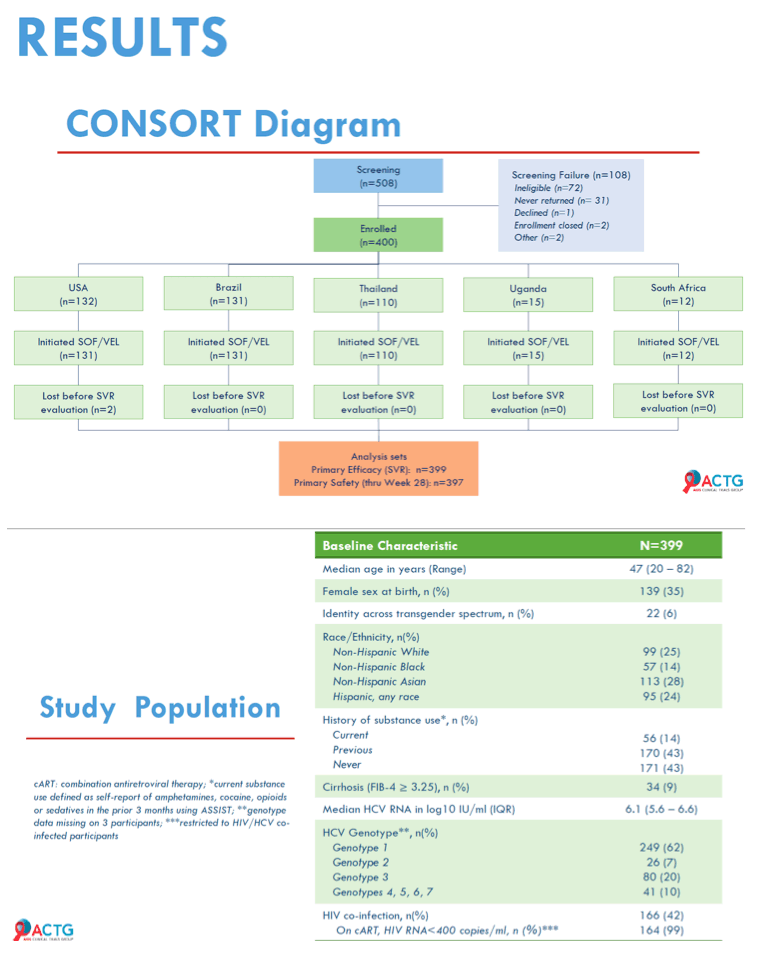
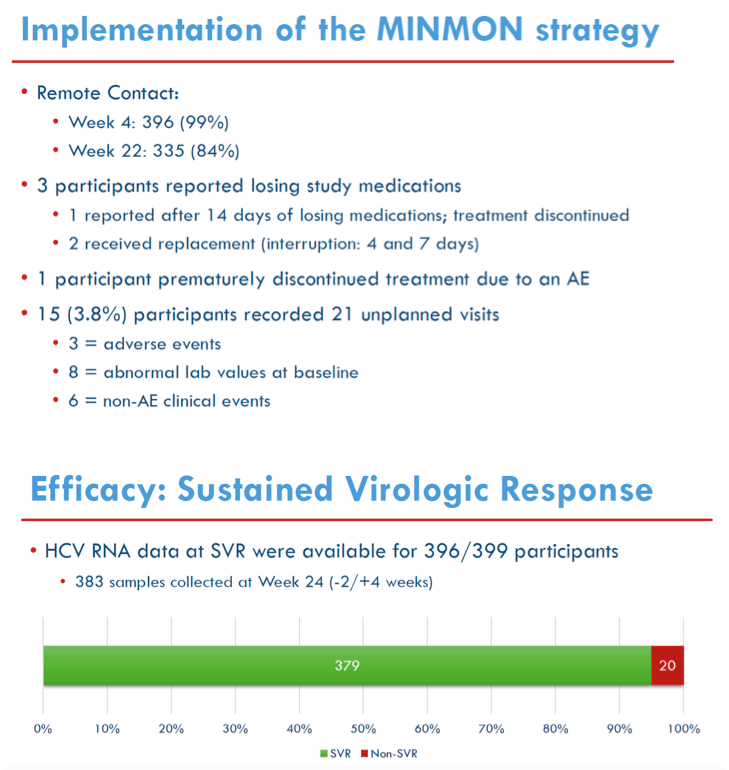
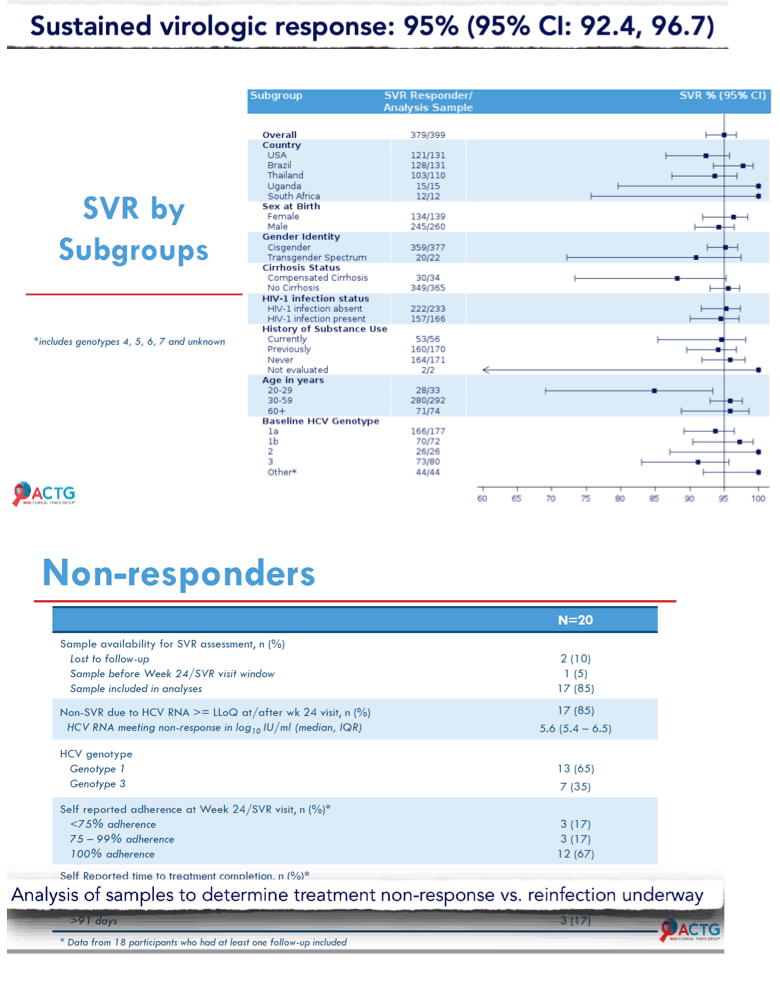
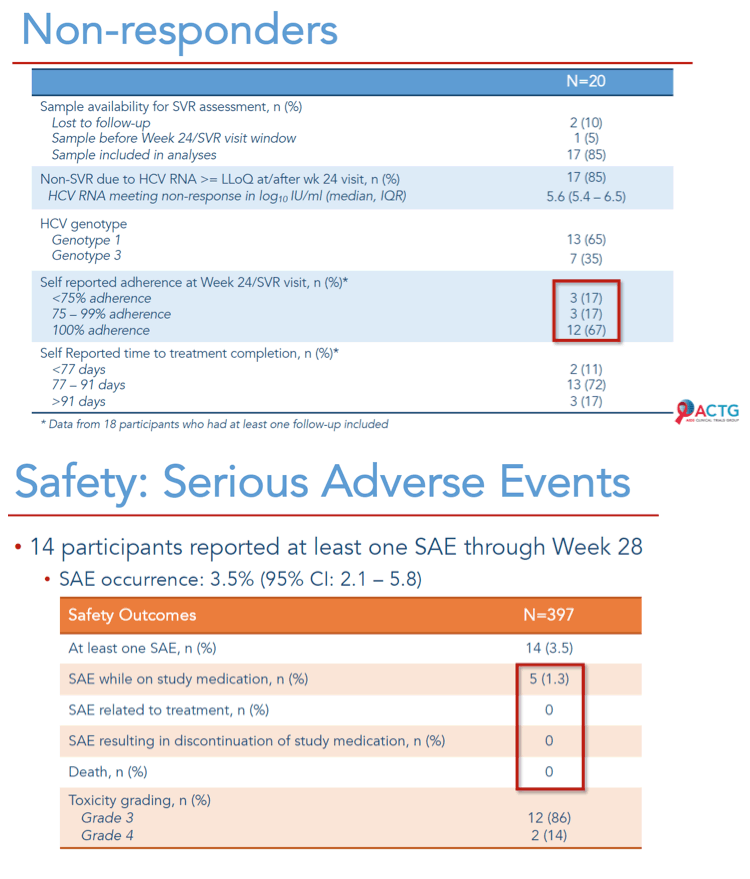
Results: 400 participants were enrolled from 10/2018-07/2019 at 38 sites in five countries across 4 continents; 399 initiated treatment. Median age was 47 years, 138 (35%) were cisgender women, 22 (6%) self-identified across the transgender spectrum, and 166 (42%) were White. At entry, 34 (9%) had compensated cirrhosis (FIB-4 ≥3.25) and 166 (42%) had HIV co-infection. Remote contact was successful at Weeks 4 and 22 for 394 (99%) and 335 (84%) participants, respectively. HCV RNA for SVR was available for 396 participants. Overall, 95% (379/399) achieved SVR (95% CI: 92.4%, 96.7%); SVR by country, biological sex, gender identity, age, cirrhosis status, HIV co-infection status and injection drug use are presented in Figure. Fifteen (3.8%) participants had unplanned visits; 3 were AE related and 6 were related to abnormalities during screening. Serious AE events through Week 24 visit were reported in 14 (3.5%) participants; none were treatment related or resulted in death.
Conclusion: In a diverse, global patient population, the MINMON approach to HCV treatment delivery was safe and achieved SVR comparable to current standards. Wider adoption of this approach coupled with innovative case- finding strategies may facilitate HCV elimination while minimizing in-person appointments and resource use.



|
| |
|
 |
 |
|
|