 |
 |
 |
| |
COST-EFFECTIVENESS OF LONG-ACTING PrEP AMONG MSM/TGW IN THE US
|
| |
| |
CROI 2021 March 6-10
Anne M . Neilan , Raphael J. Landovitz , Mylinh H. Le , Beatriz Grinsztejn , Kenneth Freedberg , Marybeth McCauley , Nattanicha Wattananimitgul , Myron S. Cohen , Andrea Ciaranello , David Paltiel , Rochelle P. Walensky
MassachusettsGeneralHospital,Boston,MA,USA, UniversityofCaliforniaLos Angeles,LosAngeles,CA,USA, InstitutedePesquisaClinicaEvandroChagas,RiodeJaneiro,Brazil, FHI360,Washington,DC,USA, UniversityofNorthCarolinaat ChapelHill,ChapelHill,NC,USA, YaleSchoolofPublicHealth,NewHaven,CT,USA
Program abstract - may be different than final slide presentation below
Background:
HIV Prevention Trials Network (HPTN) 083 demonstrated superior efficacy
of long-acting injectable cabotegravir (CAB-LA) compared to oral tenofovir disoproxil fumarate/emtricitabine (F/TDF) for HIV pre-exposure prophylaxis (PrEP). CAB-LA cost may be higher than that of generic F/TDF. We projected the clinical benefit of CAB-LA vs. F/TDF and estimated the cost at which CAB-LA would be cost-effective.
Methods:
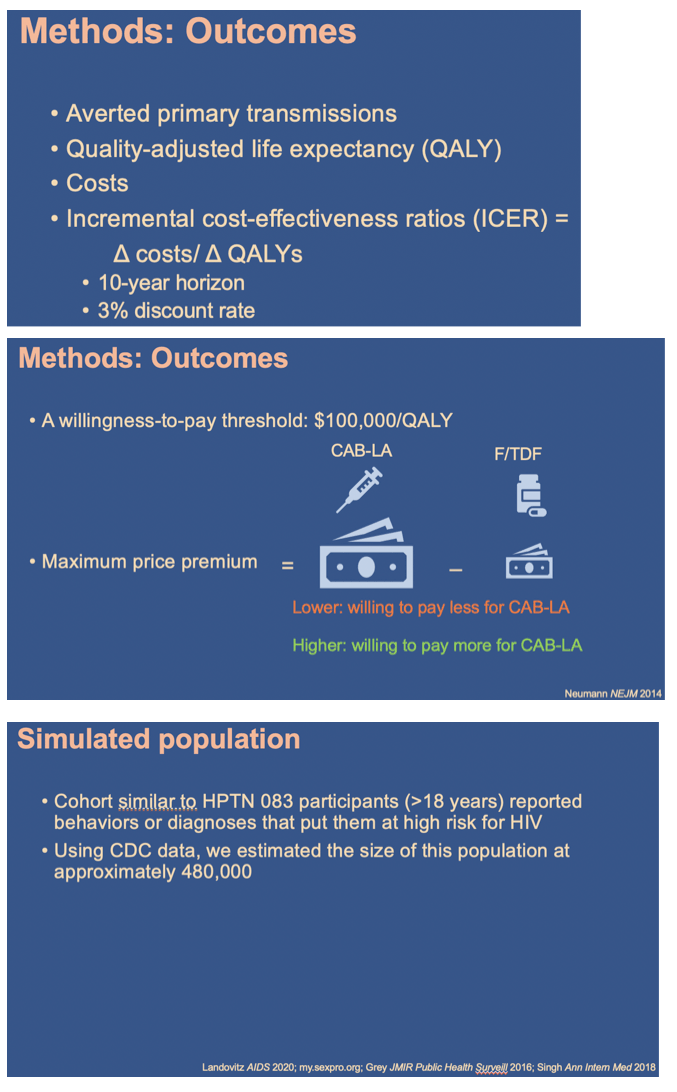
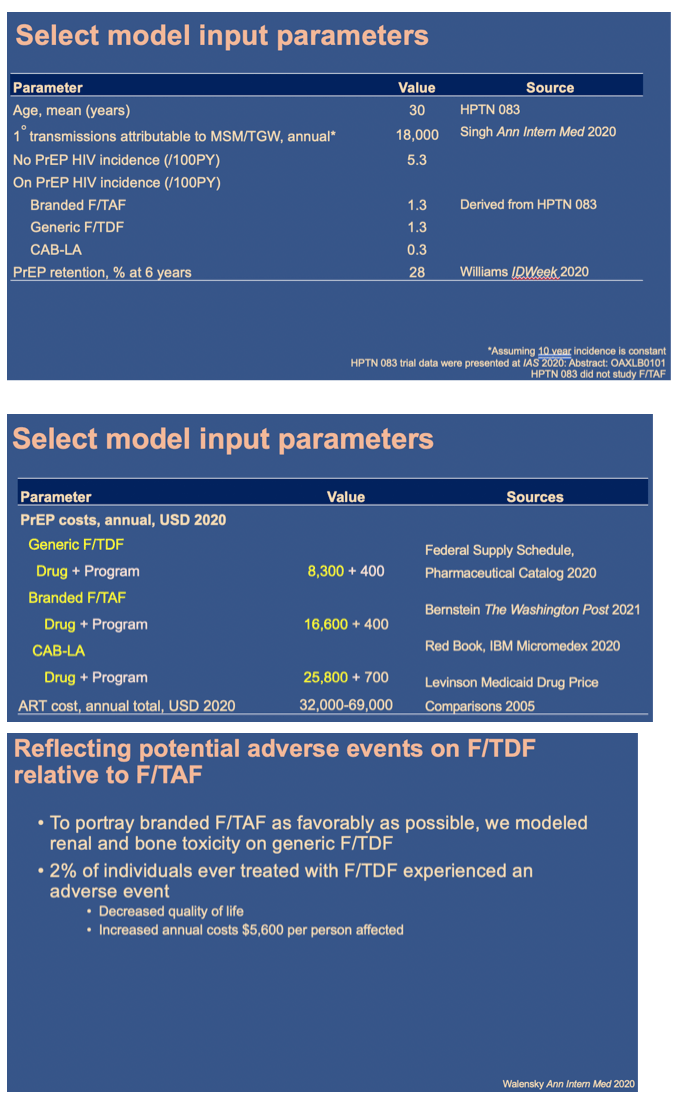
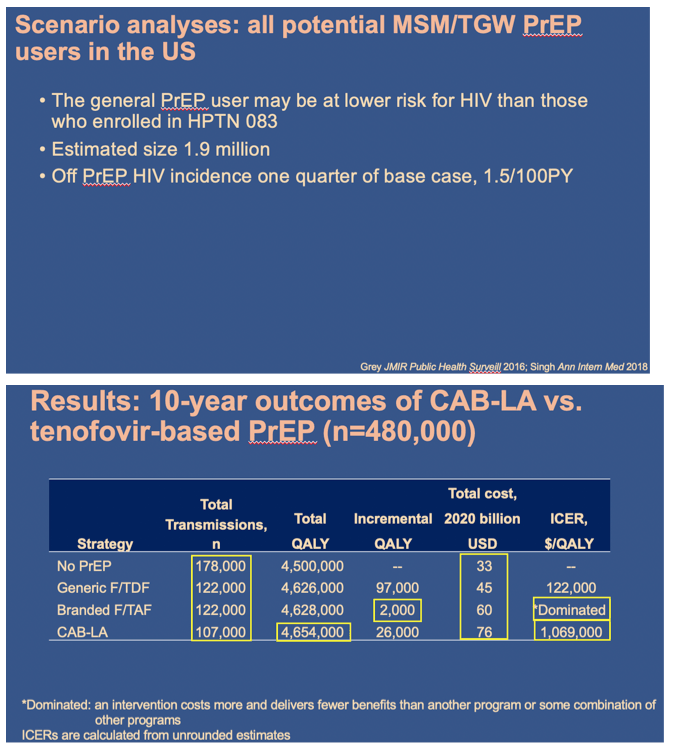
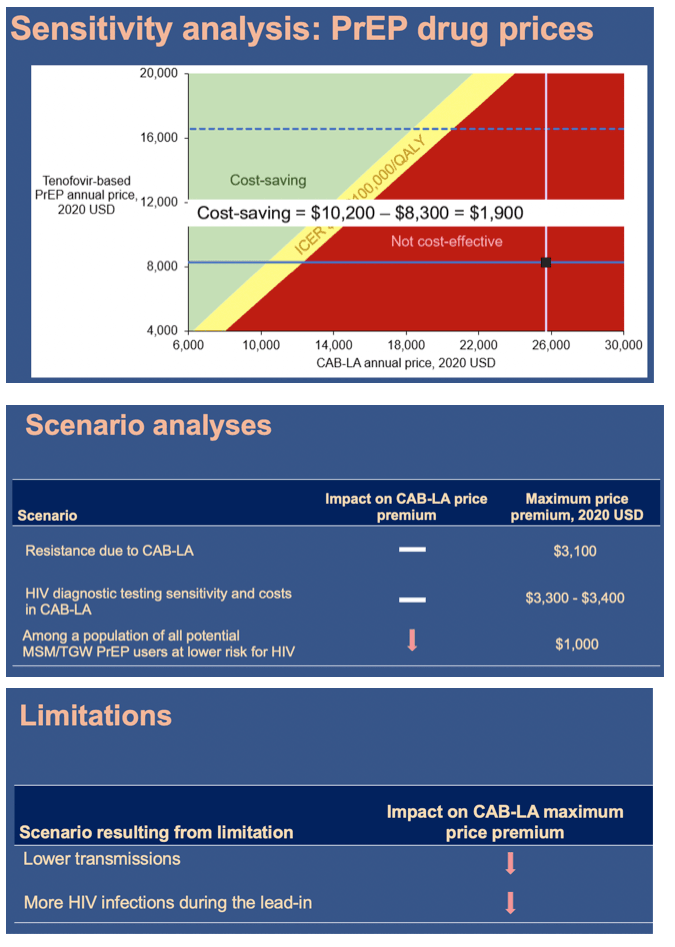
Using the Cost-Effectiveness of Preventing AIDS Complications (CEPAC) simulation model, we examined 2 strategies: generic F/TDF (or branded F/TAF) and CAB-LA among high-risk men who have sex with men and transgender women (MSM/TGW, i.e. trial-eligible) starting PrEP in the US (n=∼476,300). We used trial and published data including: HIV incidence (off PrEP: 5.32/100PY; F/ TDF (and F/TAF): 1.33/100PY; CAB-LA: 0.26/100PY); HIV transmissions off-PrEP attributable to high-risk MSM/TGW: 17,800/year (yr); 62% 6yr-PrEP retention. We assumed constant incidence and annual transmissions. Annual costs were: generic F/TDF $8,400 (branded F/TAF $16,900); CAB-LA $28,000, and ART: $24,500-$39,600. Projected outcomes included HIV transmissions averted, quality-adjusted life-years (QALYs), costs, and incremental cost-effectiveness ratios (ICERs, $/QALY) over 10 yrs. We used a willingness-to-pay threshold of $100,000/QALY. In sensitivity analysis, we varied PrEP costs and transmissions/ yr. We also examined providing CAB-LA to all PrEP-eligible MSM/TGW (n=∼1,905,300) - not just those at high risk - with HIV incidence off PrEP: 1.54/100PY and HIV transmissions: 19,700/yr.
Results:
In the base case, compared to generic F/TDF (or branded F/TAF), CAB-LA increased life expectancy by 37,000 QALYs (37,000 QALYs) and costs by $36.78B ($20.14B), leading to an ICER of $994,000/QALY ($544,000/QALY, Table). CAB-LA would be cost-effective compared to F/TDF or F/TAF over 10yrs at a maximum price premium over F/TDF (F/TAF) of $700/yr ($1,800/yr). When offered to all PrEP-eligible MSM/TGW, CAB-LA would be cost-effective over 10yrs at a maximum price premium of $200/yr (vs. F/TDF) or $500/yr (vs. F/TAF).
Conclusion:
The superiority of long-acting injectable PrEP notwithstanding, the presence of highly effective alternatives limits the additional price difference that payers should be willing to pay for CAB-LA.


|
| |
|
 |
 |
|
|