 |
 |
 |
| |
WEIGHT AND LIPID CHANGES IN PHASE 3
CABOTEGRAVIR AND RILPIVIRINE LONG-ACTING TRIALS
|
| |
| |
CROI 2021 March 6-10 Reported by Jules Levin
Parul Patel1, Ronald D'Amico1, Shanker Thiagarajah2, Sterling Wu3, Emilie Elliot4, Joseph W. Polli1, Ojesh Upadhyay3, Rodica Van Solingen-Ristea5, Chloe Orkin6, E. Turner Overton7, Susan Swindells8, Jean A. Van Wyk9, Matthew Bosse1, Vani Vannappagari1
1ViiV Healthcare, Research Triangle Park, NC, USA, 2GlaxoSmithKline, Uxbridge, UK, 3GlaxoSmithKline, Collegeville, PA, USA, 4ViiV Healthcare, Madrid, Spain, 5Janssen Research and Development, Beerse, Belgium, 6Queen Mary University of London, London, UK, 7University of Alabama at Birmingham, Birmingham, AL, USA 8University of Nebraska Medical Center,Omaha, NE,USA, 9ViiV Healthcare, Brentford, UK
Background: Weight gain and metabolic alterations have been reported with integrase strand transfer inhibitor (InSTI)-based antiretroviral (ARV) regimens. Long-acting (LA) cabotegravir (CAB), an InSTI, and rilpivirine (RPV), a non- nucleoside reverse transcriptase inhibitor, constitute a highly effective 2-drug regimen administered intramuscularly monthly or every 2 months for the maintenance of virologic suppression. Weight and lipid changes over 48 weeks in adults with virologic suppression receiving CAB+RPV LA in Phase 3/3b clinical trials are presented.
Methods: Data in participants naïve to CAB+RPV LA and randomized to CAB+RPV LA every 4 weeks (Q4W), every 8 weeks (Q8W), or oral comparator ARV therapy (CAR) through 48 weeks were pooled from the ATLAS, FLAIR, and ATLAS-2M studies. Demographics and baseline characteristics were collected for each group and changes in weight, BMI, and lipids from baseline to Week 48 were analyzed.
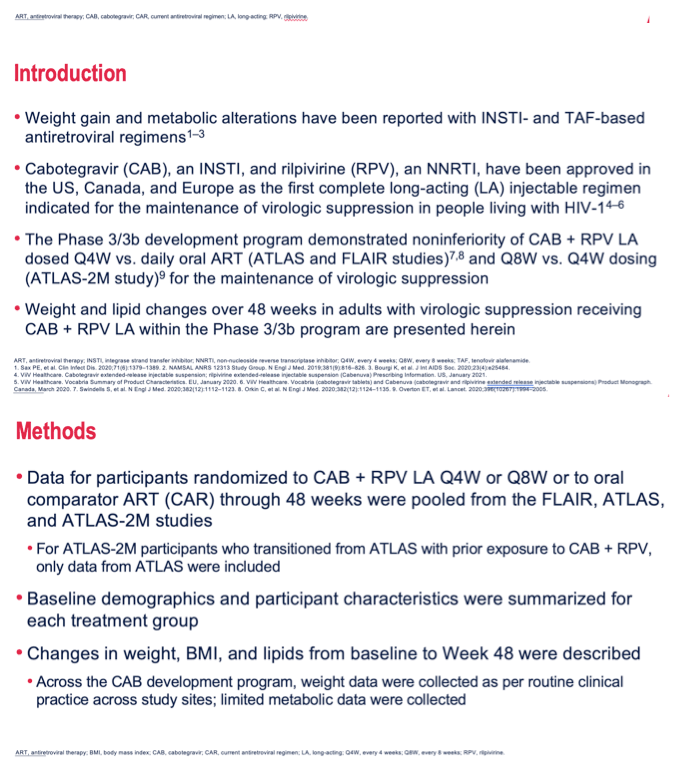
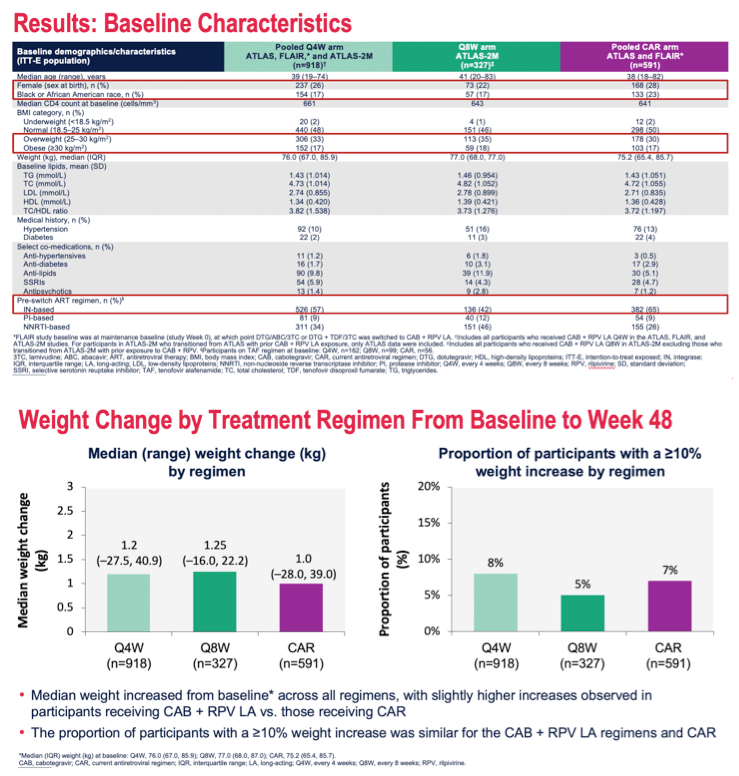
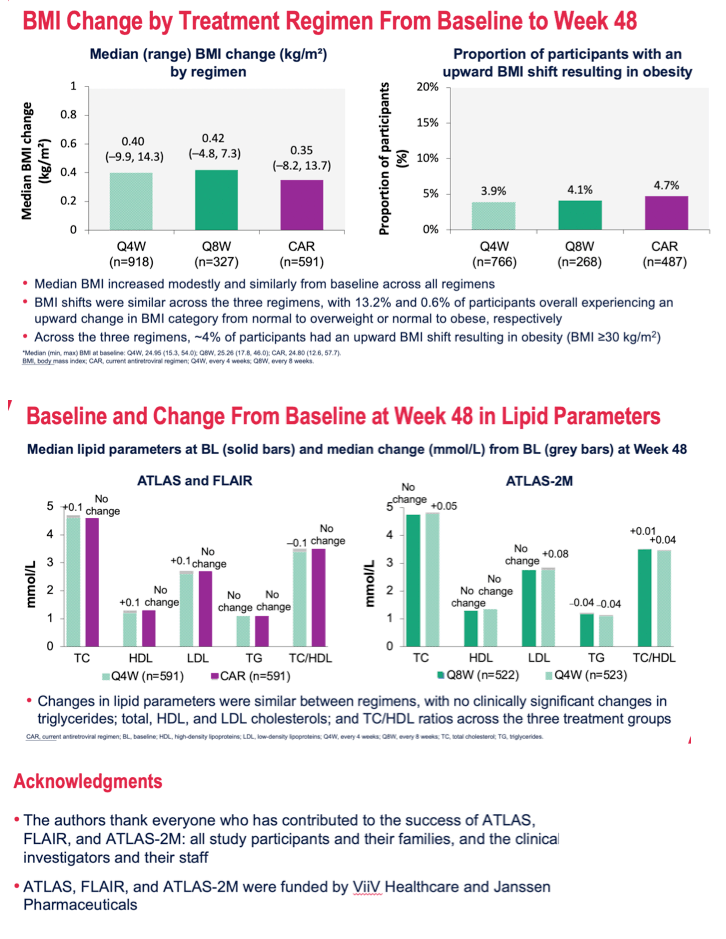
Results: Participants' baseline characteristics are summarized in Table 1. Median (range) change in weight from baseline to Week 48 was 1.20 kg (-27.5, 40.9) in Q4W, 1.25 kg (-16.0, 22.2) in Q8W, and 1.00 kg (-28.0, 39.0) in the CAR groups. ≥10% weight increase occurred in 77 (8%) participants in Q4W, 15 (5%) in Q8W, and 39 (7%) in the CAR groups. Median (range) change in BMI was 0.40 kg/m2 (-9.9, 14.3) in Q4W, 0.42 kg/m2 (-4.8, 7.3) in Q8W, and 0.35 kg/m2 (-8.2, 13.7) in the CAR groups. 13.4% (59/440) of participants in Q4W, 14.6% (22/151) in Q8W, and 13.8% (41/298) in the CAR groups underwent an upward shift in BMI category from normal, resulting in 3.9% (30/766, Q4W), 4.1% (11/268, Q8W), and 4.7% (23/487, CAR) of participants developing clinical obesity (BMI >30 kg/m2). There were no clinically significant changes in triglycerides; total, HDL, and LDL cholesterols; and TC/HDL ratios among the 3 treatment groups.
Conclusion: In this pooled analysis, changes in weight and lipid parameters over 48 weeks were modest and similar, respectively, in participants receiving CAB+RPV LA Q4W or Q8W compared to CAR. Since InSTI-associated weight changes have only recently emerged, collection of weight data across the CAB development program was not standardized at sites and limited metabolic data were collected. Future and on-going studies will further characterize potential InSTI-associated weight gain and metabolic perturbations.

|
| |
|
 |
 |
|
|