 |
 |
 |
| |
IMPAACT 2014 24-WEEK PK AND SAFETY OF DORAVIRINE/3TC/TDF IN ADOLESCENTS WITH HIV-1
|
| |
| |
CROI 2021 March 6-10 Reported by Jules Levin
Ann J . Melvin1, Brookie Best2, Petronella Muresan3, Sarah Pasyar3, Hedy Teppler4, Kelly Yee4, Katie McCarthy5, Rachel Scheckter5, Hong Wan4, Lina De Montigny4, Linda Aurpibul6, Pradthana Ounchanum7, Avy Violari8, Nicole Tobin9, Ellen Townley10
1University of Washington, Seattle, WA, USA, 2University of California San Diego, San Diego, CA, USA, 3Harvard TH Chan School of Public Health, Boston, MA, USA, 4Merck & Co, Inc, Kenilworth, NJ, USA, 5FHI 360, Durham, NC, USA, 6Chiang Mai University, Chiang Mai, Thailand, 7Chiang Rai Prachanukroh Hospital, Chiang Rai, Thailand, 8University of the Witwatersrand, Johannesburg, South Africa, 9University of California Los Angeles, Los Angeles, CA, USA, 10National Institutes of Health, Rockville, MD, USA
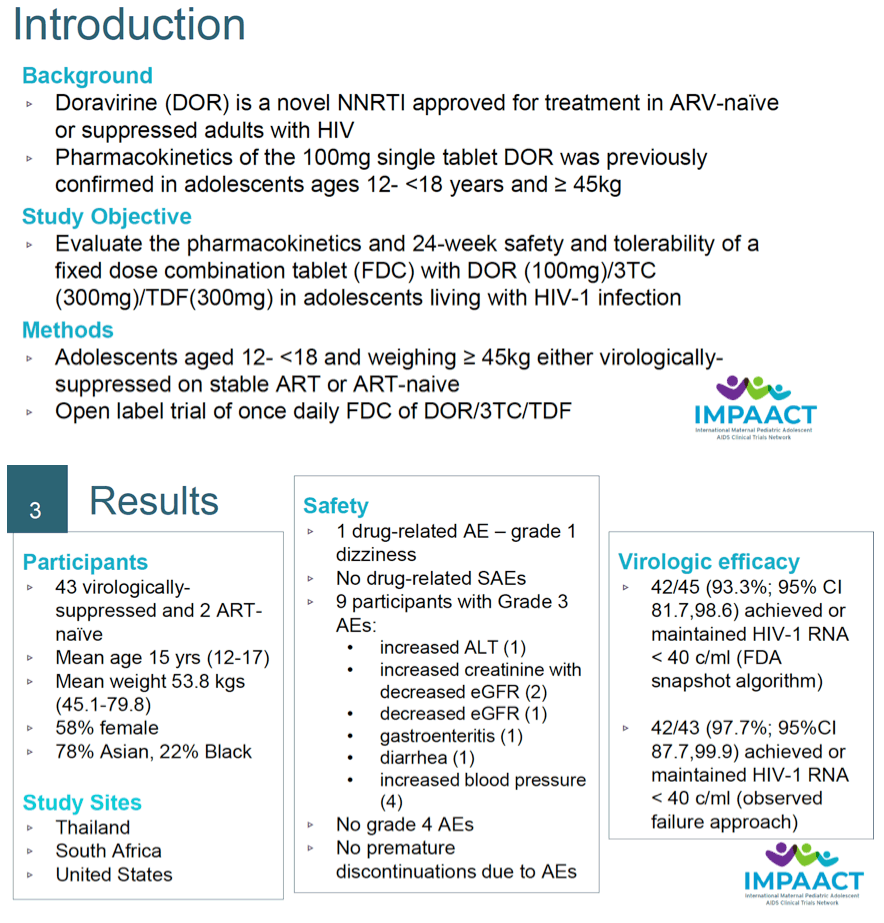
Background: Doravirine (DOR) is a novel non-nucleoside reverse transcriptase inhibitor (NNRTI) active against both wild-type HIV-1 and the most common NNRTI-resistant variants. DOR, alone or as a fixed dose combination (FDC) of DOR/lamivudine (3TC)/tenofovir disoproxil fumarate (TDF), is approved for treatment in antiretroviral-naïve and virologically-suppressed adults with HIV-1 infection. IMPAACT 2014 investigated the pharmacokinetics (PK) and safety of DOR as a component of the FDC in adolescents with HIV-1 through 24 weeks. The 100mg dose of DOR evaluated in this study was previously confirmed based on 2-week safety and single dose PK in adolescents >45kg.
Methods: Adolescents with HIV-1, between the ages of 12 and 18 years and weighing at least 45 kg who were antiretroviral therapy (ART)-naïve or virologically-suppressed on stable ART, were enrolled into an open-label trial evaluating the once daily FDC tablet regimen of DOR 100mg, 3TC 300mg, and TDF 300mg. Safety, virologic and PK data were evaluated through Week 24 of therapy.
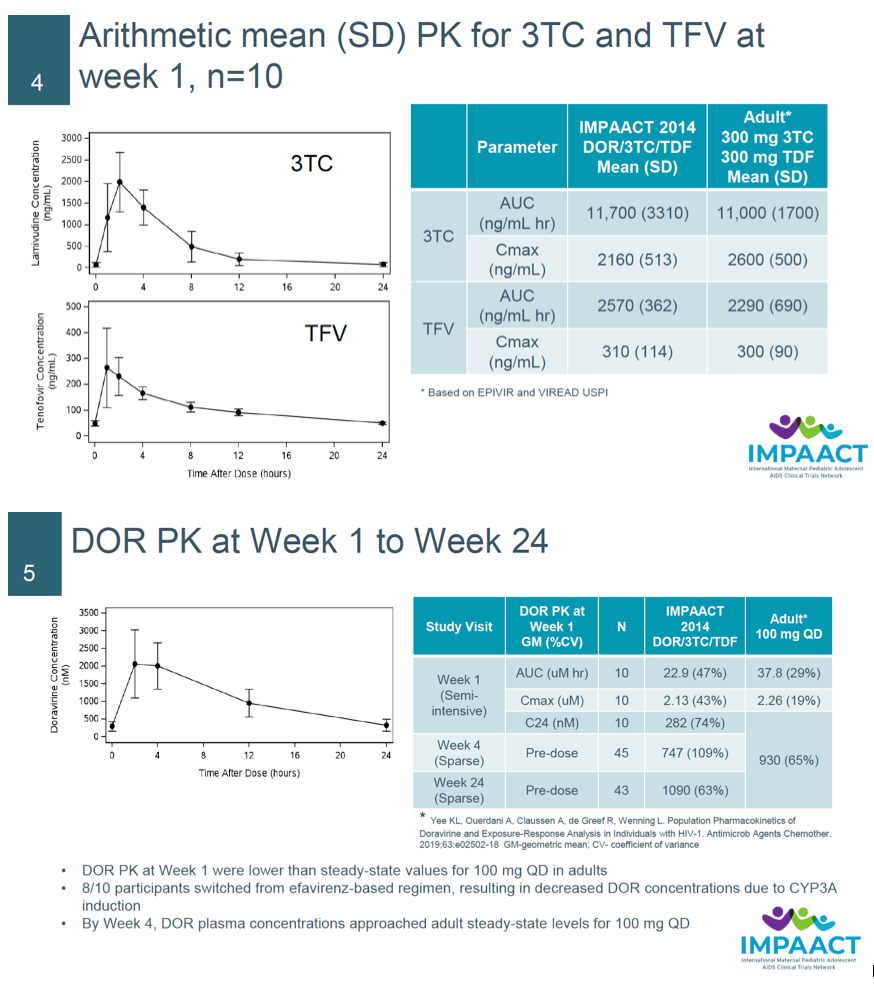
Results: Forty-five adolescents (43 virologically-suppressed on stable ART and 2 ART-naïve at enrollment) were evaluated. Mean age of the participants was 15 years (range 12-17 years) with a mean weight of 53.8 kg (range 45.1 - 79.8 kg). Overall, the FDC of DOR/3TC/TDF was well tolerated through 24 weeks. There was a low incidence of drug-related AEs (2.2% with 95% CI [0.1-11.8]), no drug- related SAEs or AEs ≥Grade 3 and no treatment discontinuation due to AEs. In the virologically-suppressed on stable ART participants there were no protocol- defined virologic failures and HIV-1 RNA <50 copies/mL was maintained at 95.3% with a 95% CI [84.2, 99.4]. One of the 2 ART-naïve participants achieved virologic suppression by Week 24 and the other experienced protocol-defined virologic failure related to adherence issues. DOR geometric mean steady state trough concentration at Week 4 was 747 nM and subsequent concentrations were above the lower efficacy bound (>560 nM) for adults with HIV receiving 100mg QD DOR. PK for 3TC and TFV from the FDC were consistent with reported PK in adults receiving each drug individually.
Conclusion: At Week 24 the PK, safety, and tolerability of DOR as an FDC of DOR/3TC/TDF in adolescents were comparable to data reported in adults. Overall virologic efficacy in the trial showed favorable antiretroviral effect comparable to data reported in adults.
|
| |
|
 |
 |
|
|