 |
 |
 |
| |
TRENDS IN TRUVADA AND DESCOVY PRESCRIPTIONS FOR PrEP IN THE UNITED STATES, 2014-2020
|
| |
| |
CROI 2021 March 6-10 Reported by Jules Levin
Karen W . Hoover1, Weiming Zhu1, Jeffrey Wiener1, Ya-Lin A. Huang1
1Centers for Disease Control and Prevention, Atlanta, GA, USA
Background: Emtricitabine and tenofovir disoproxil fumarate (F/TDF) was the only PrEP drug available in the United States until the FDA approved emtricitabine and tenofovir alafenamide (F/TAF) for PrEP on October 3,
2019. Both drugs are safe and effective for PrEP. In 2018, the U.S. healthcare system spent $2.1 billion on PrEP for 18% of persons with a PrEP indication. Less expensive generic formulations of F/TDF are expected in late 2021 when emtricitabine's patent expires. We estimated trends in the use of these drugs and switching from F/TDF to F/TAF.
Methods: We analyzed data from the IQVIA prescription database to estimate the number of persons prescribed F/TDF or F/TAF for PrEP by calendar quarter from January 2014 through June 2020. During October 1, 2019 through June 30, 2020, we estimated the proportion of new PrEP users prescribed F/TDF or F/TAF. Among a cohort of persons prescribed F/TDF for PrEP by October 3, 2019 and with at least one PrEP prescription after that date, we assessed the proportion who switched to F/TAF. Using a multivariable Poisson regression model, we estimated the probability of switching from F/TDF to F/TAF vs. continuing on F/ TDF for PrEP by patient demographic characteristics.
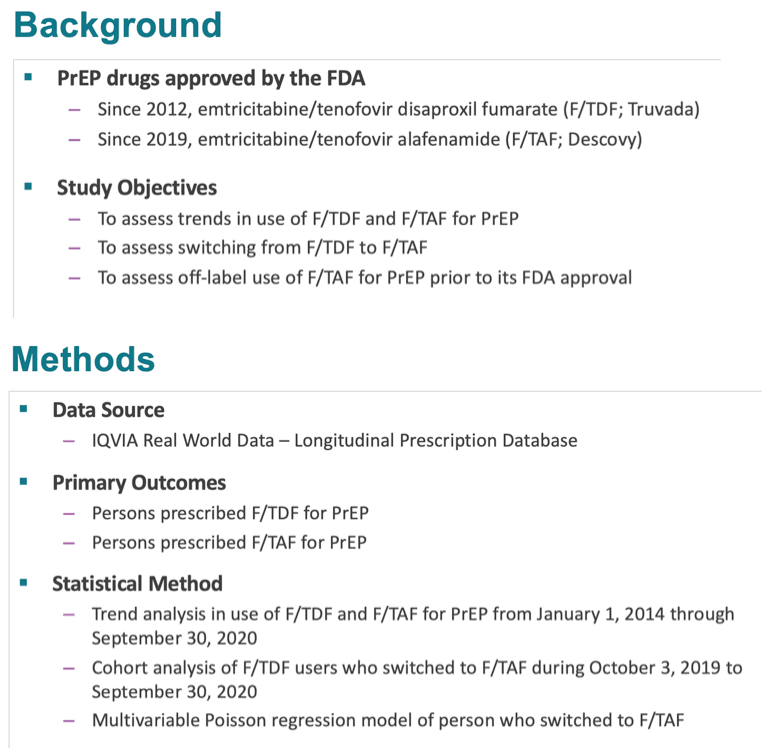
Results: The number of PrEP users prescribed F/TAF increased from 2,637 in the third quarter (Q3) of 2019 to 75,979 in the second quarter of 2020. The number of PrEP users prescribed F/TDF decreased starting in Q3 2019 (Figure).
During October 1, 2019 to June 30, 2020, 43,316 (38.1%) of 113,559 new PrEP users were prescribed F/TAF.
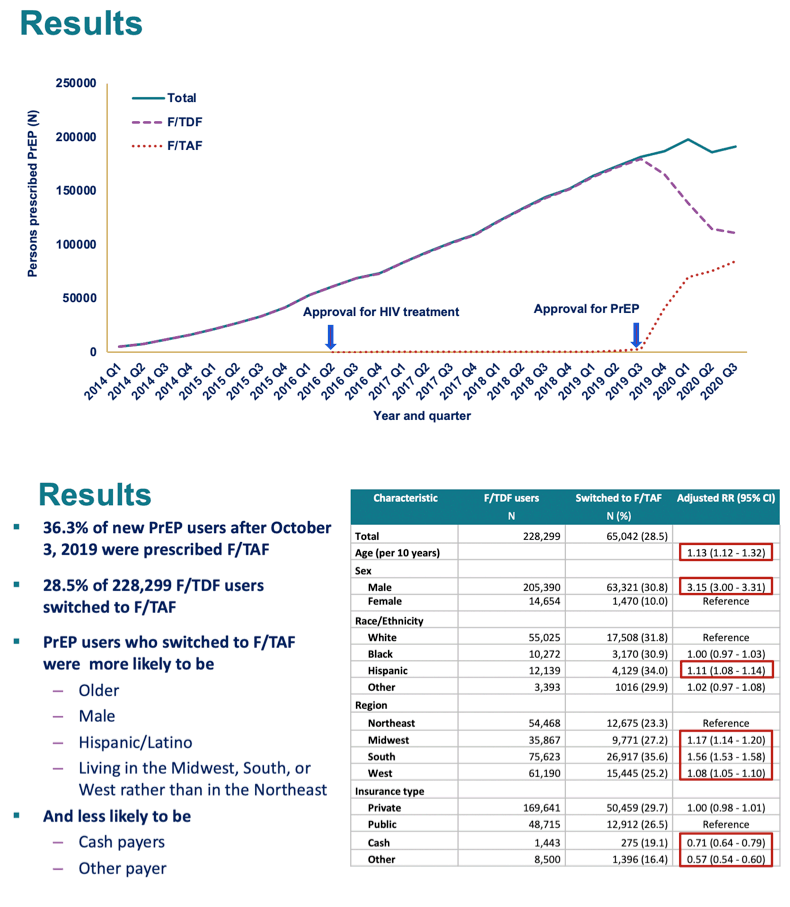
Among a cohort of 205,248 persons prescribed F/TDF before October 3, 2019 and with at least one PrEP prescription after that date, 57,059 (27.2%) switched to F/TAF.
In a multivariable regression model, the adjusted probability of switching from F/TDF to F/TAF vs. continuing on F/TDF was higher in older persons (aRR 1.14, 95% CI 1.13- 1.15 for each 10 year age increase), persons privately insured vs. publicly insured (aRR 1.29, 95% CI 1.26 - 1.32), and persons living in the South, West or Midwest a vs. the Northeast (aRR 1.52, 95% CI 1.49 - 1.55), Latino (see graph).
Conclusion: Since approval of F/TAF in early October 2019, many PrEP users have initiated F/TAF or switched from F/TDF to F/TAF. As new patented and generic PrEP drugs become available, monitoring their use can help understand implications for U.S. healthcare system expenditures. Clinicians might consider prescribing less expensive options that can result in lower healthcare system expenditures for PrEP.


|
| |
|
 |
 |
|
|