 |
 |
 |
| |
CLINICAL EVALUATION OF DRUG INTERACTIONS
WITH ORAL LENACAPAVIR AND PROBE DRUGS
|
| |
| |
CROI 2021 March 6-10 reported by Jules Levin
Rebecca Begley1, Justin Lutz1, Hadas Dvory-Sobol1, Steve West1, Kristin Kawata1, John Ling1, Martin Rhee1, Polina German1
1Gilead Sciences, Inc, Foster City, CA, USA
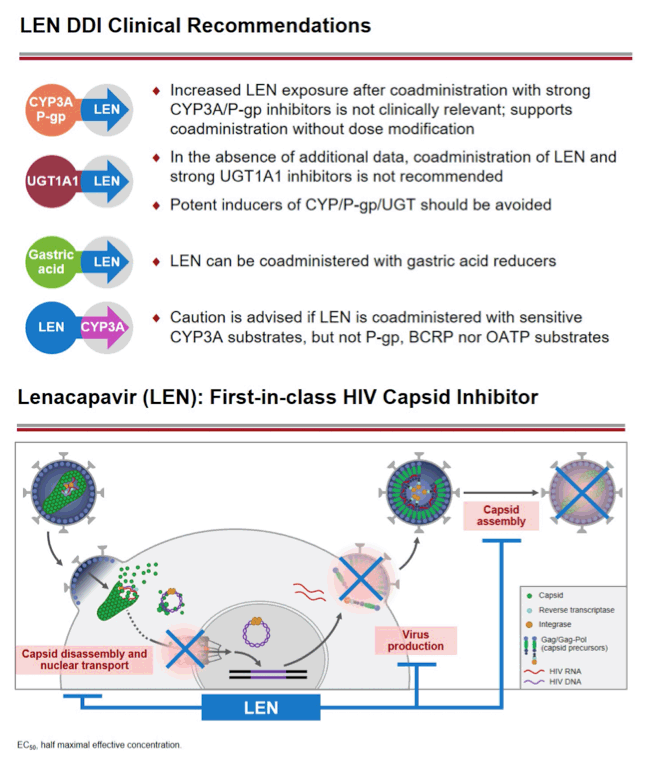
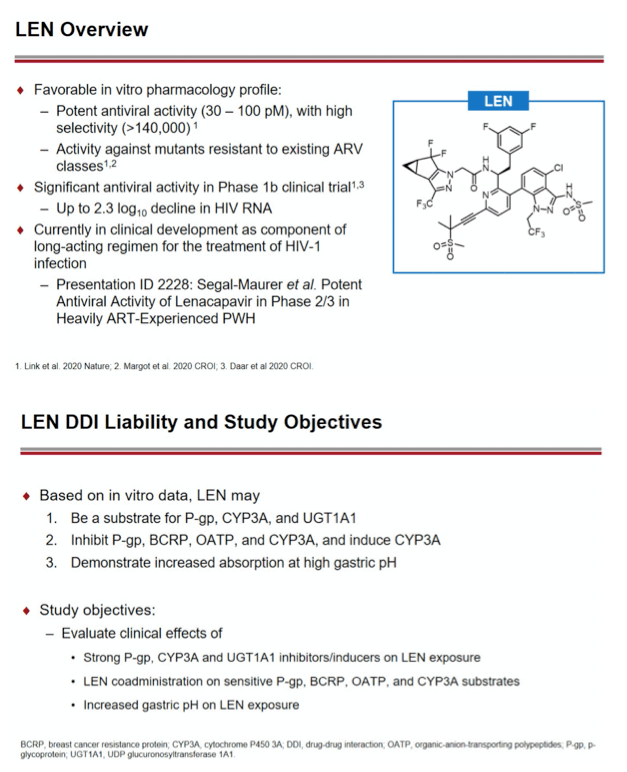
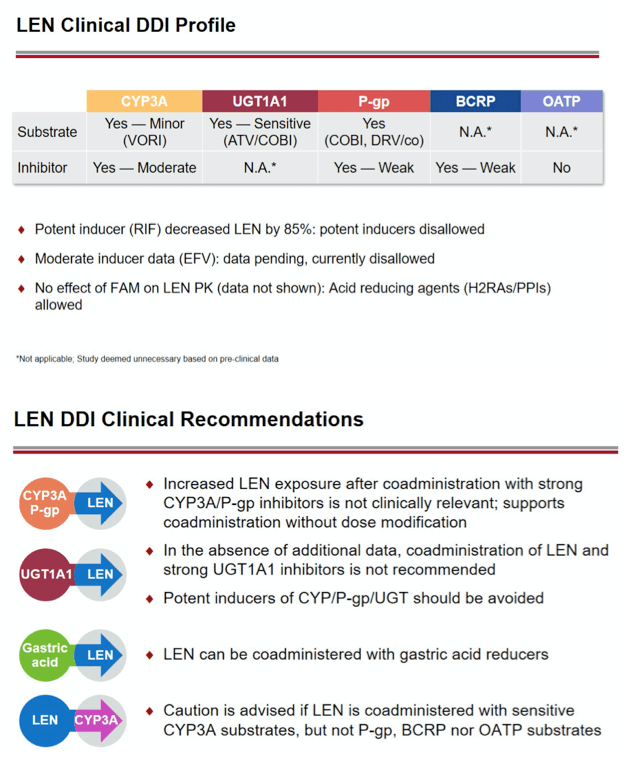
Background: Lenacapavir (LEN; GS-6207) is a potent, selective, first-in-class, multi-stage inhibitor of HIV-1 capsid function in clinical development as a long- acting agent for treatment and prevention of HIV-1 infection. Based on in vitro data, LEN is a substrate for P-gp, CYP3A, and UGT1A1, may inhibit P-gp, BCRP, OATP, and CYP3A, and may induce CYP3A. Also, LEN exhibits pH-dependent solubility (higher solubility at pH ≥6). In this Phase 1 study, we evaluated LEN drug interactions using cobicistat (COBI) +/- darunavir (DRV) as inhibitors of CYP3A/P-gp, voriconazole (VORI) as an inhibitor of CYP3A, rifampin (RIF) as an inducer of CYP/P-gp/UGT, and famotidine (FAM) as a gastric acid-reducing agent. The perpetrator interaction potential was evaluated using pitavastatin (PIT; OATP substrate), rosuvastatin (ROS; BCRP/OATP substrate), tenofovir alafenamide (TAF; P-gp substrate), and midazolam (MDZ; CYP3A substrate).
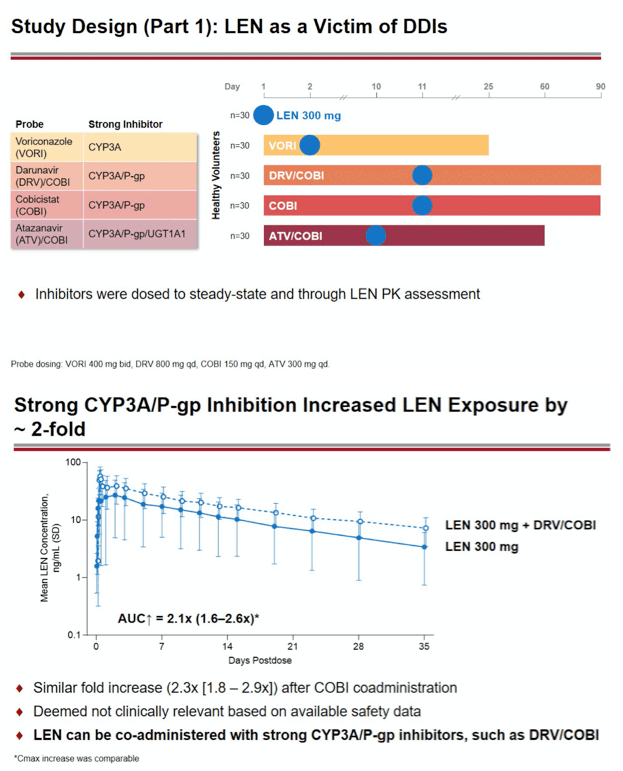
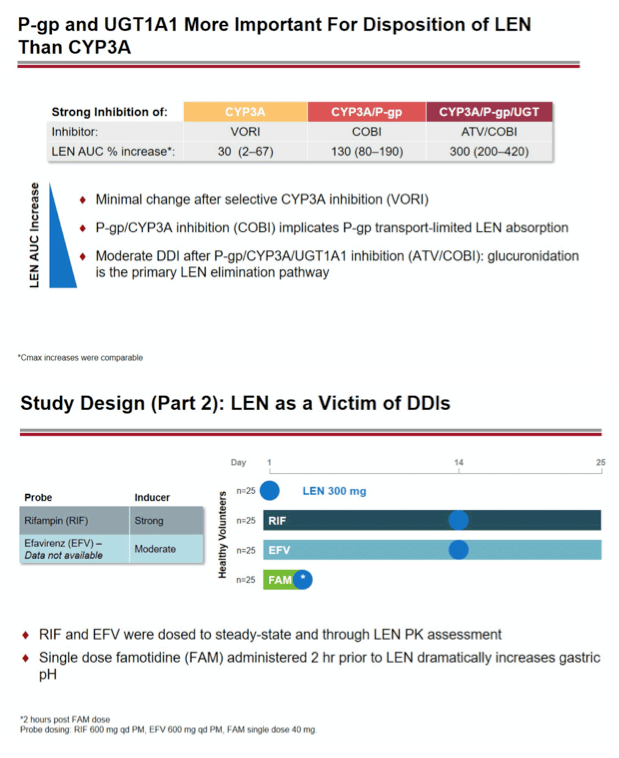
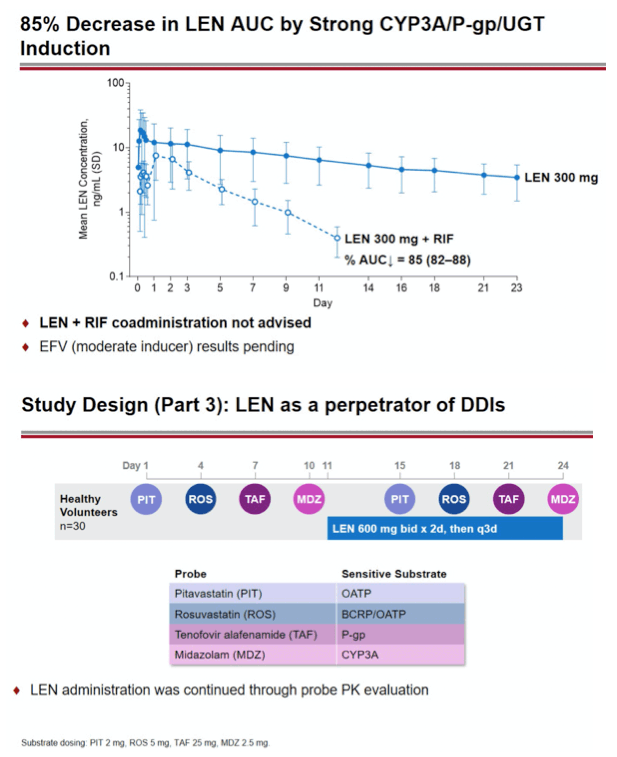
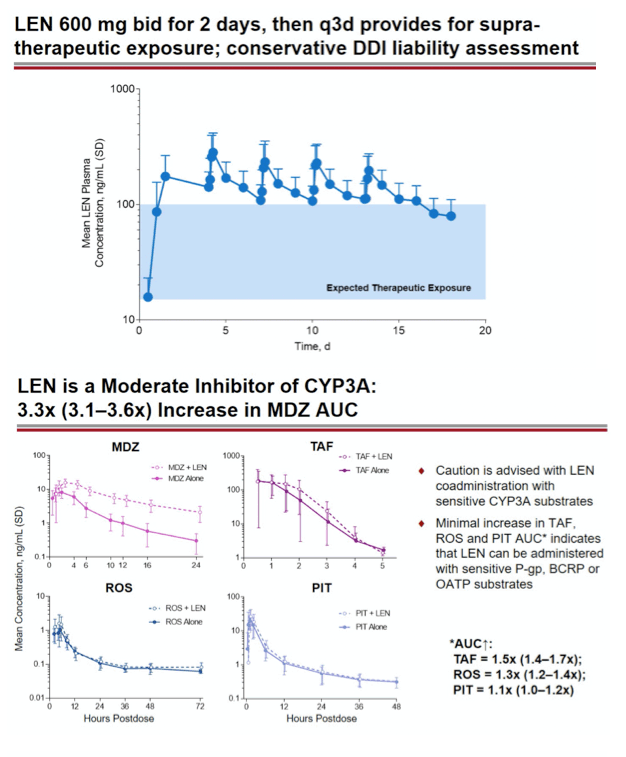
Methods: In separate cohorts of healthy participants (parallel study design), single oral doses of LEN (300mg) were given with and without COBI (150mg QD, Days 1-35), DRV/COBI (800mg/150mg QD, Days 1-35), VORI (400mg BID x 1 day, then 200mg BID Days 2-25), RIF (600mg QD, Days 1-25), and FAM (40mg single dose 2 h prior to LEN). In another cohort, single doses of PIT (2mg), ROS (5 mg), TAF (25mg), and MDZ (2.5mg) were given prior to and simultaneous with LEN 600mg BID x2 days, then Q3 days to rapidly achieve clinically relevant LEN exposure.
Results: Geometric-least squares means ratio (GLSM) and 90% confidence intervals (90% CI) for AUC∞ () and Cmax (•) for combination vs substrate alone were estimated by linear mixed effect modeling: Of 223 total participants, none had serious or Grade 4 adverse events (AEs). One participant had a Grade 3 AE of elevated transaminase while receiving LEN + ROS, which led to study drug discontinuation. Most AEs were mild in severity.
Conclusion: Consistent with in vitro data, these results confirm that LEN is a substrate of both CYP3A and P-gp. The magnitude of inhibition via COBI, DRV/COBI, and VORI is not considered to be clinically relevant, supporting coadministration of LEN with potent inhibitors of CYP3A and P-gp without dose modification. However, potent inducers of CYP/P-gp/UGT, such as RIF, should be avoided. LEN is not affected by gastric acid reducers. As a perpetrator, LEN would be considered a moderate inhibitor of CYP3A, and a weak inhibitor of P-gp, BCRP, with no effect on OATP. Overall, LEN has a limited drug interaction potential.

|
| |
|
 |
 |
|
|